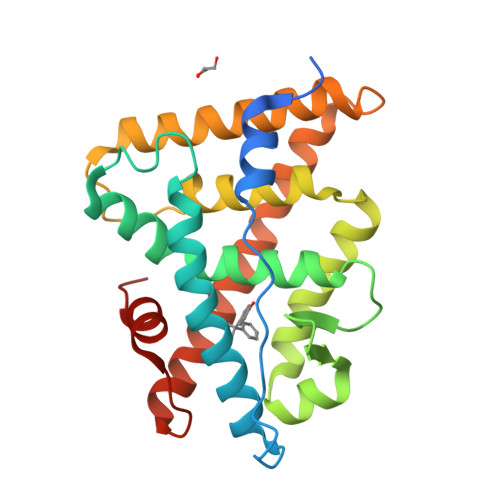
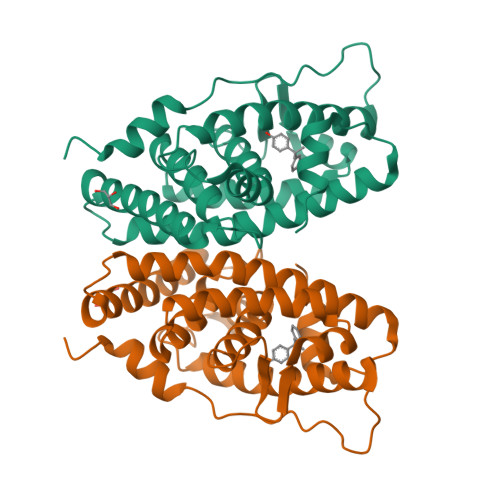
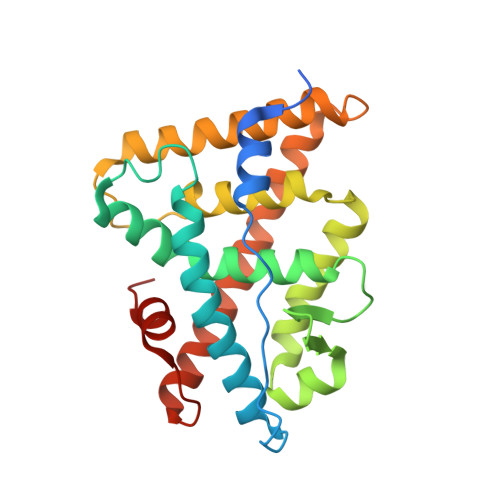
ERRgamma tethers strongly bisphenol A and 4-alpha-cumylphenol in an induced-fit manner
Matsushima, A., Teramoto, T., Okada, H., Liu, X., Tokunaga, T., Kakuta, Y., Shimohigashi, Y.(2008) Biochem Biophys Res Commun 373: 408-413
- PubMed: 18582436
- DOI: https://doi.org/10.1016/j.bbrc.2008.06.050
- Primary Citation of Related Structures:
2ZAS, 2ZBS - PubMed Abstract:
A receptor-binding assay and X-ray crystal structure analysis demonstrated that the endocrine disruptor bisphenol A (BPA) strongly binds to human estrogen-related receptor gamma (ERRgamma). BPA is well anchored to the ligand-binding pocket, forming hydrogen bonds with its two phenol-hydroxyl groups. In this study, we found that 4-alpha-cumylphenol lacking one of its phenol-hydroxyl groups also binds to ERRgamma very strongly. The 2.0 A crystal structure of the 4-alpha-cumylphenol/ERRgamma complex clearly revealed that ERRgamma's Leu345-beta-isopropyl plays a role in the tight binding of 4-alpha-cumylphenol and BPA, rotating in a back-and-forth induced-fit manner.
Organizational Affiliation:
Laboratory of Structure-Function Biochemistry, Department of Chemistry, The Research-Education Centre of Risk Science, Faculty and Graduate School of Sciences, Kyushu University, Fukuoka 812-8581, Japan.