Rounding up: Engineering 12-Membered Rings from the Cyclic 11-Mer TRAP
Heddle, J.G., Yokoyama, T., Yamashita, I., Park, S.Y., Tame, J.R.H.(2006) Structure 14: 925-933
- PubMed: 16698553
- DOI: https://doi.org/10.1016/j.str.2006.03.013
- Primary Citation of Related Structures:
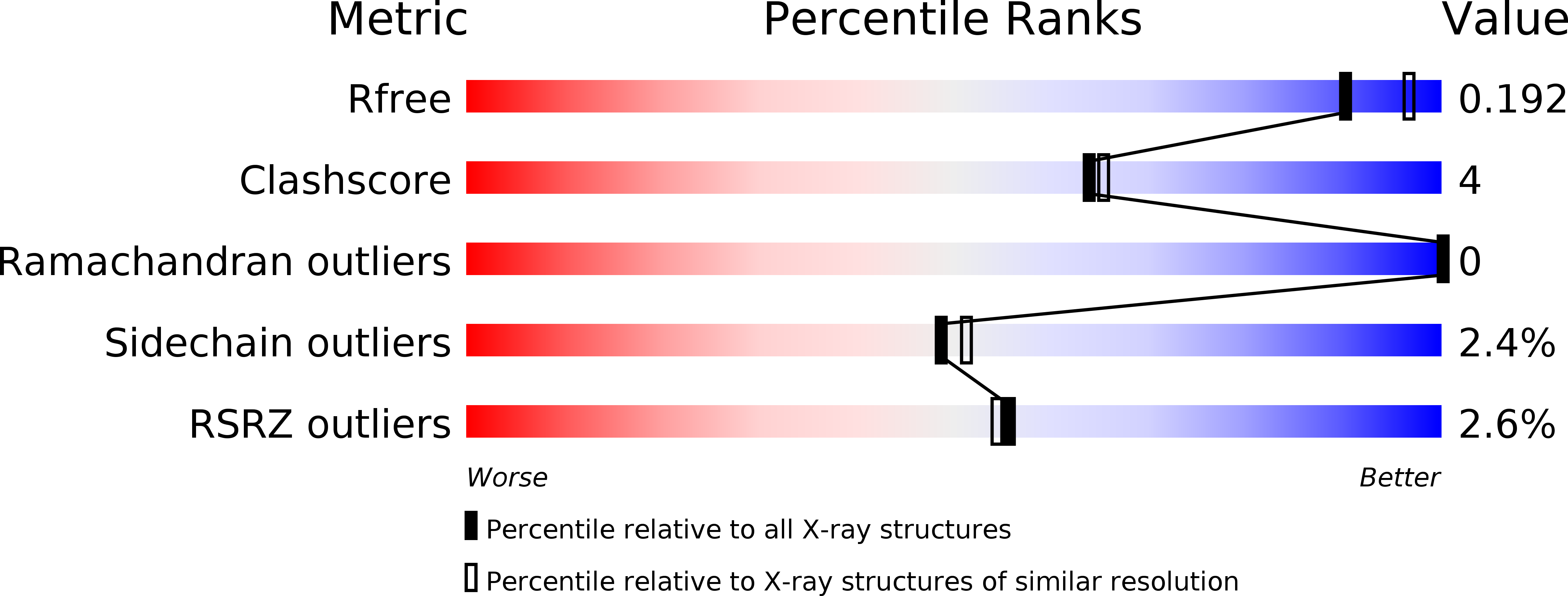
2EXS, 2EXT - PubMed Abstract:
The protein TRAP (trp RNA binding attenuation protein) forms a highly thermostable ring-shaped 11-mer. By linking in tandem two, three, or four DNA sequences encoding TRAP monomers, we have engineered new rings that consist of 12 TRAP subunits and bind 12 ligand molecules. The hydrogen bonding pattern and buried surface area within and between subunits are essentially identical between the 11-mer and 12-mer crystal structures. Why do the artificial proteins choose to make single 12-mer rings? The 12-mer rings are highly sterically strained by their peptide linkers and far from thermostable. That proteins choose to adopt a strained conformation of few subunits rather than an unstrained one with 11 subunits demonstrates the importance of entropic factors in controlling protein-protein interactions in general.
Organizational Affiliation:
Protein Design Laboratory, Yokohama City University, Tsurumi, Suehiro 1-7-29, Yokohama 230-0045, Japan. jgh4@tsurumi.yokohama-cu.ac.jp