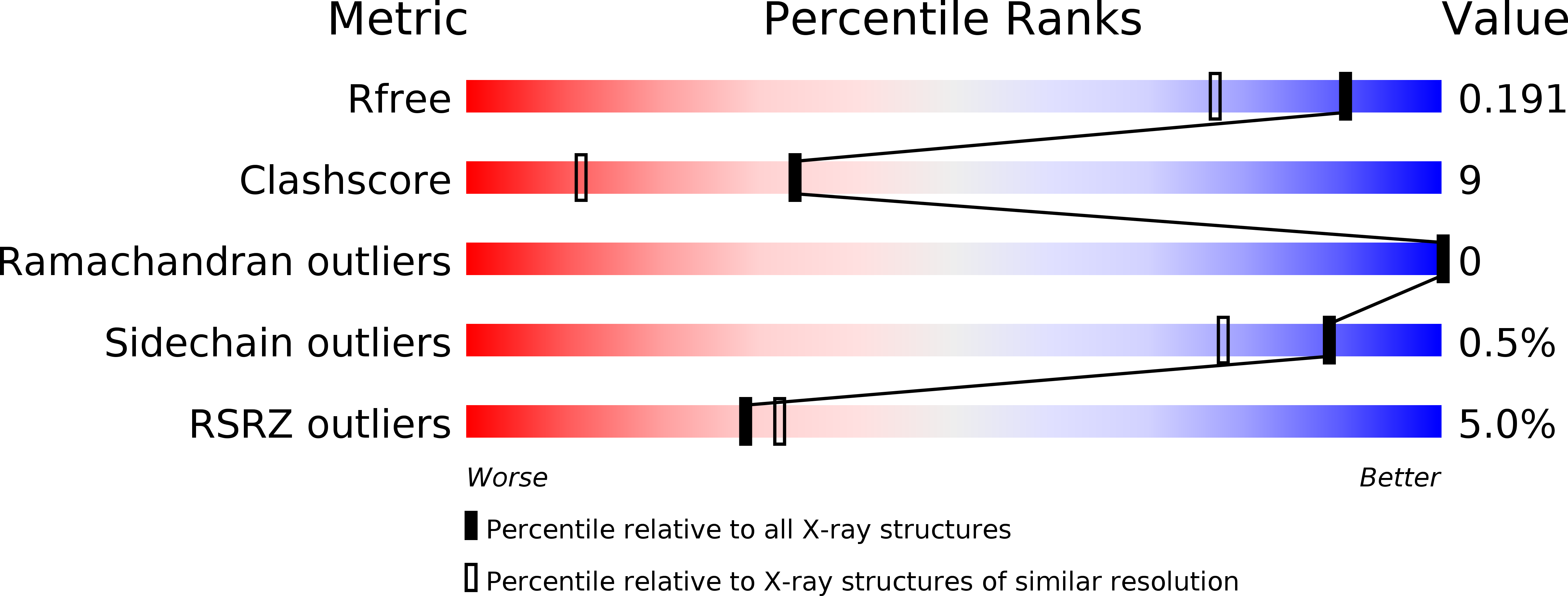
In Silico Identification and Crystal Structure Validation of Caspase-3 Inhibitors without a P1 Aspartic Acid Moiety.
Ganesan, R., Jelakovic, S., Mittl, P.R., Caflisch, A., Grutter, M.G.(2011) Acta Crystallogr Sect F Struct Biol Cryst Commun 67: 842
- PubMed: 21821879
- DOI: https://doi.org/10.1107/S1744309111018604
- Primary Citation of Related Structures:
2XYG, 2XYH, 2XYP - PubMed Abstract:
Using a fragment-based docking procedure, several small-molecule inhibitors of caspase-3 were identified and tested and the crystal structures of three inhibitor complexes were determined. The crystal structures revealed that one inhibitor (NSC 18508) occupies only the S1 subsite, while two other inhibitors (NSC 89167 and NSC 251810) bind only to the prime part of the substrate-binding site. One of the major conformational changes observed in all three caspase-3-inhibitor complexes is a rotation of the Tyr204 side chain, which blocks the S2 subsite. In addition, the structural variability of the residues shaping the S1-S4 as well as the S1' subsites supports an induced-fit mechanism for the binding of the inhibitors in the active site. The high-resolution crystal structures reported here provide novel insights into the architecture of the substrate-binding site, which might be useful for the design of more potent caspase inhibitors.
Organizational Affiliation:
Department of Biochemistry, University of Zürich, Zürich, Switzerland.