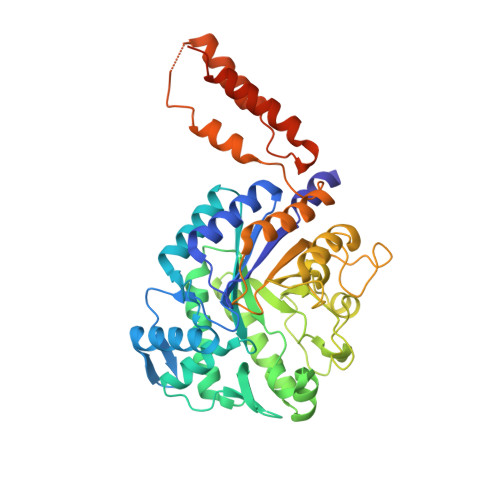
Inhibition of the Pneumococcal Virulence Factor Strh and Molecular Insights Into N-Glycan Recognition and Hydrolysis.
Pluvinage, B., Higgins, M.A., Abbott, D.W., Robb, C., Dalia, A.B., Deng, L., Weiser, J.N., Parsons, T.B., Fairbanks, A.J., Vocadlo, D.J., Boraston, A.B.(2011) Structure 19: 1603
- PubMed: 22078560
- DOI: https://doi.org/10.1016/j.str.2011.08.011
- Primary Citation of Related Structures:
2YL5, 2YL6, 2YL8, 2YL9, 2YLA, 2YLL - PubMed Abstract:
The complete degradation of N-linked glycans by the pathogenic bacterium Streptococcus pneumoniae is facilitated by the large multimodular cell wall-attached exo-β-D-N-acetylglucosaminidase StrH. Structural dissection of this virulence factor using X-ray crystallography showed it to have two structurally related glycoside hydrolase family 20 catalytic domains, which displayed the expected specificity for complex N-glycans terminating in N-acetylglucosamine but exhibited unexpected differences in their preferences for the substructures present in these glycans. The structures of the two catalytic domains in complex with unhydrolyzed substrates, including an N-glycan possessing a bisecting N-acetylglucosamine residue, revealed the specific architectural features in the active sites that confer their differential specificities. Inhibitors of StrH are demonstrated to be effective tools in modulating the interaction of StrH with components of the host, such as the innate immune system. Overall, new structural and functional insight into a carbohydrate-mediated component of the pneumococcus-host interaction is provided.
Organizational Affiliation:
Biochemistry and Microbiology, University of Victoria, Victoria, BC V8W 3P6, Canada.