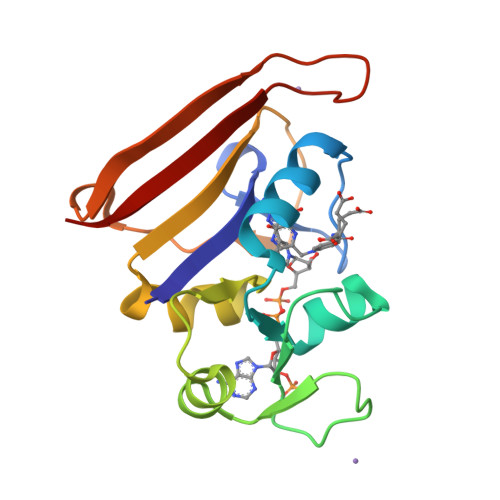
A dynamic knockout reveals that conformational fluctuations influence the chemical step of enzyme catalysis.
Bhabha, G., Lee, J., Ekiert, D.C., Gam, J., Wilson, I.A., Dyson, H.J., Benkovic, S.J., Wright, P.E.(2011) Science 332: 234-238
- PubMed: 21474759
- DOI: https://doi.org/10.1126/science.1198542
- Primary Citation of Related Structures:
3QL0, 3QL3 - PubMed Abstract:
Conformational dynamics play a key role in enzyme catalysis. Although protein motions have clear implications for ligand flux, a role for dynamics in the chemical step of enzyme catalysis has not been clearly established. We generated a mutant of Escherichia coli dihydrofolate reductase that abrogates millisecond-time-scale fluctuations in the enzyme active site without perturbing its structural and electrostatic preorganization. This dynamic knockout severely impairs hydride transfer. Thus, we have found a link between conformational fluctuations on the millisecond time scale and the chemical step of an enzymatic reaction, with broad implications for our understanding of enzyme mechanisms and for design of novel protein catalysts.
Organizational Affiliation:
Department of Molecular Biology and Skaggs Institute for Chemical Biology, The Scripps Research Institute, La Jolla, CA 92037, USA.