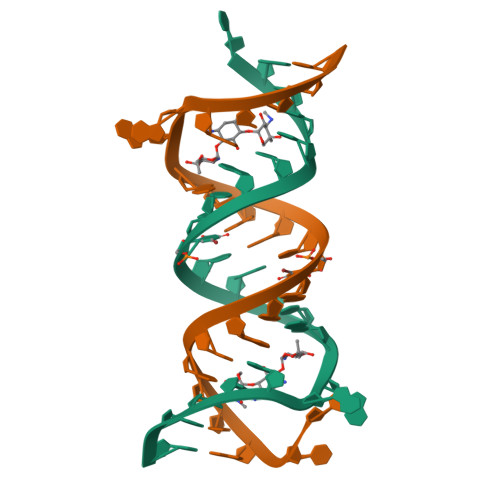
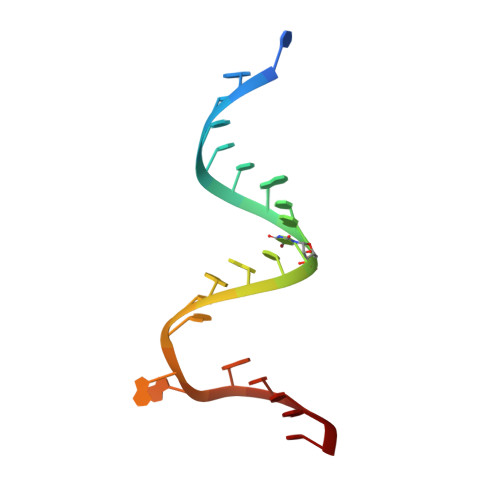
A structural basis for the antibiotic resistance conferred by an A1408G mutation in 16S rRNA and for the antiprotozoal activity of aminoglycosides
Kondo, J.(2012) Angew Chem Int Ed Engl 51: 465-468
- PubMed: 22110016
- DOI: https://doi.org/10.1002/anie.201106084
- Primary Citation of Related Structures:
3TD0, 3TD1 - PubMed Abstract:
Resistance explained: The crystal structures of the ribosomal decoding A site with an A1408G antibiotic-resistance mutation were solved in the presence and absence of the aminoglycoside geneticin (see structure, geneticin carbon framework in yellow). These structures show how bacteria acquire high-level resistance against aminoglycosides by the mutation.
Organizational Affiliation:
Department of Materials and Life Sciences, Faculty of Science and Technology, Sophia University, 7-1 Kioi-cho, Chiyoda-ku, 102-8554 Tokyo, Japan. j.kondo@sophia.ac.jp