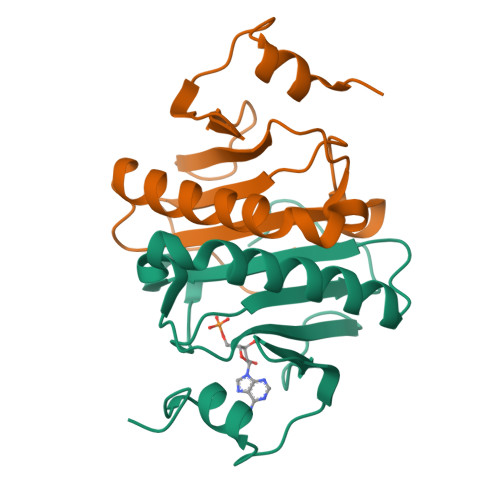
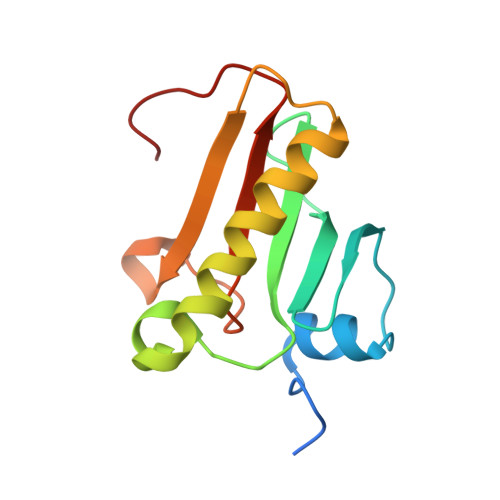
A new crystal form of human histidine triad nucleotide-binding protein 1 (hHINT1) in complex with adenosine 5'-monophosphate at 1.38 A resolution.
Dolot, R., Ozga, M., Wlodarczyk, A., Krakowiak, A., Nawrot, B.(2012) Acta Crystallogr Sect F Struct Biol Cryst Commun 68: 883-888
- PubMed: 22869114
- DOI: https://doi.org/10.1107/S1744309112029491
- Primary Citation of Related Structures:
3TW2 - PubMed Abstract:
Histidine triad nucleotide-binding protein 1 (HINT1) represents the most ancient and widespread branch of the histidine triad protein superfamily. HINT1 plays an important role in various biological processes and has been found in many species. Here, the structure of the human HINT1-adenosine 5'-monophosphate (AMP) complex at 1.38 Å resolution obtained from a new monoclinic crystal form is reported. The final structure has R(cryst) = 0.1207 (R(free) = 0.1615) and the model exhibits good stereochemical quality. Detailed analysis of the high-resolution data allowed the details of the protein structure to be updated in comparison to the previously published data.
Organizational Affiliation:
Department of Bioorganic Chemistry, Centre of Molecular and Macromolecular Studies, Sienkiewicza 112, 90-363 Łódź, Poland. rdolot@cbmm.lodz.pl