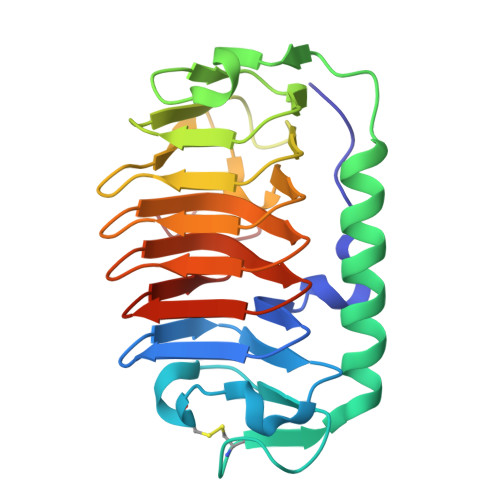
Hyperactive antifreeze protein from an Antarctic sea ice bacterium Colwellia sp. has a compound ice-binding site without repetitive sequences.
Hanada, Y., Nishimiya, Y., Miura, A., Tsuda, S., Kondo, H.(2014) FEBS J 281: 3576-3590
- PubMed: 24938370
- DOI: https://doi.org/10.1111/febs.12878
- Primary Citation of Related Structures:
3WP9 - PubMed Abstract:
Antifreeze proteins (AFPs) are structurally diverse macromolecules that bind to ice crystals and inhibit their growth to protect the organism from injuries caused by freezing. An AFP identified from the Antarctic bacterium Colwellia sp. strain SLW05 (ColAFP) is homologous to AFPs from a wide variety of psychrophilic microorganisms. To understand the antifreeze function of ColAFP, we have characterized its antifreeze activity and determined the crystal structure of this protein. The recombinant ColAFP exhibited thermal hysteresis activity of approximately 4 °C at a concentration of 0.14 mm, and induced rapid growth of ice crystals in the hexagonal direction. Fluorescence-based ice plane affinity analysis showed that ColAFP binds to multiple planes of ice, including the basal plane. These observations show that ColAFP is a hyperactive AFP. The crystal structure of ColAFP determined at 1.6 Å resolution revealed an irregular β-helical structure, similar to known homologs. Mutational and molecular docking studies showed that ColAFP binds to ice through a compound ice-binding site (IBS) located at a flat surface of the β-helix and the adjoining loop region. The IBS of ColAFP lacks the repetitive sequences that are characteristic of hyperactive AFPs. These results suggest that ColAFP exerts antifreeze activity through a compound IBS that differs from the characteristic IBSs shared by other hyperactive AFPs. This study demonstrates a novel method for protection from freezing by AFPs in psychrophilic microorganisms. Structural data for ColAFP have been submitted to the Protein Data Bank (PDB) under accession number 3WP9.
Organizational Affiliation:
Graduate School of Life Science, Hokkaido University, Sapporo, Japan; Bioproduction Research Institute, National Institute of Advanced Industrial Science and Technology, Sapporo, Japan.