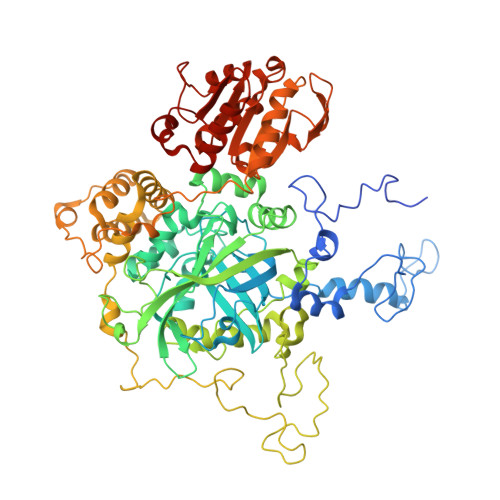
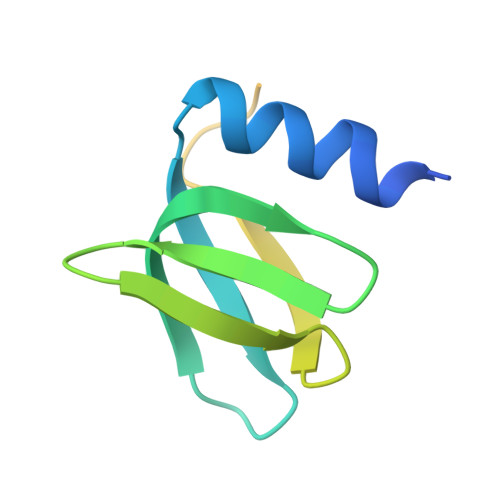
Post-Transcriptional Regulator Hfq Binds Catalase HPII: Crystal Structure of the Complex
Yonekura, K., Watanabe, M., Kageyama, Y., Hirata, K., Yamamoto, M., Maki-Yonekura, S.(2013) PLoS One 8: e78216-e78216
- PubMed: 24223139
- DOI: https://doi.org/10.1371/journal.pone.0078216
- Primary Citation of Related Structures:
3VU3 - PubMed Abstract:
We report a crystal structure of Hfq and catalase HPII from Escherichia coli. The post-transcriptional regulator Hfq plays a key role in the survival of bacteria under stress. A small non-coding RNA (sRNA) DsrA is required for translation of the stationary phase sigma factor RpoS, which is the central regulator of the general stress response. Hfq facilitates efficient translation of rpoS mRNA, which encodes RpoS. Hfq helps in the function of other specific proteins involved in RNA processing, indicating its versatility in the cell. However, structural information regarding its interactions with partners is missing. Here we obtained crystals of Hfq and HPII complexes from cell lysates following attempts to overexpress a foreign membrane protein. HPII is one of two catalases in E. coli and its mRNA is transcribed by an RNA polymerase holoenzyme containing RpoS, which in turn is under positive control of small non-coding RNAs and of the RNA chaperone Hfq. This sigma factor is known to have a pronounced effect on the expression of HPII. The crystal structure reveals that a Hfq hexamer binds each subunit of a HPII tetramer. Each subunit of the Hfq hexamer exhibits a unique binding mode with HPII. The hexamer of Hfq interacts via its distal surface. The proximal and distal surfaces are known to specifically bind different sRNAs, and binding of HPII could affect Hfq function. Hfq-HPII complexation has no effect on catalase HPII activity.
Organizational Affiliation:
Biostructural Mechanism Laboratory, RIKEN SPring-8 Center, Harima Institute, Sayo, Hyogo, Japan.