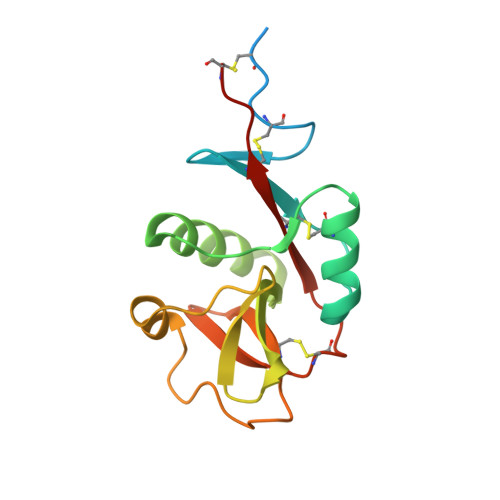
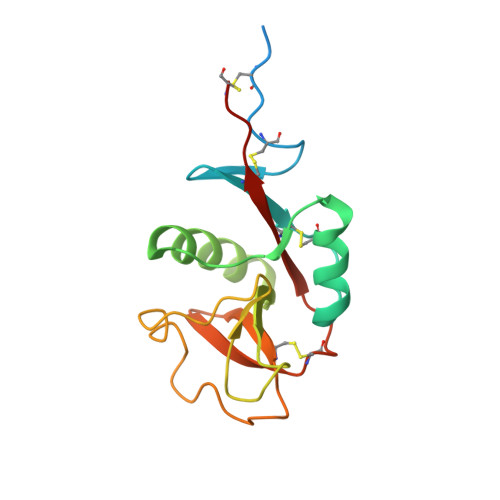
Structural Basis for Selective Recognition of Endogenous and Microbial Polysaccharides by Macrophage Receptor Sign-R1.
Silva-Martin, N., Bartual, S.G., Ramirez-Aportela, E., Chacon, P., Park, C.G., Hermoso, J.A.(2014) Structure 22: 1595
- PubMed: 25450767
- DOI: https://doi.org/10.1016/j.str.2014.09.001
- Primary Citation of Related Structures:
3ZHG, 4C9F, 4CAJ, 4CDH - PubMed Abstract:
SIGN-R1 is a principal receptor for microbial polysaccharides uptake and is responsible for C3 fixation via an unusual complement activation pathway on splenic marginal zone macrophages. In these macrophages, SIGN-R1 is also involved in anti-inflammatory activity of intravenous immunoglobulin by direct interaction with sialylated Fcs. The high-resolution crystal structures of SIGN-R1 carbohydrate recognition domain and its complexes with dextran sulfate or sialic acid, and of the sialylated Fc antibody provide insights into SIGN-R1’s selective recognition of a-2,6-sialylated glycoproteins. Unexpectedly, an additional binding site has been found in the SIGNR1 carbohydrate recognition domain, structurally separate from the calcium-dependent carbohydrate-binding site. This secondary binding site could bind repetitive molecular patterns, as observed in microbial polysaccharides, in a calcium-independent manner. These two binding sites may allow SIGNR1 to simultaneously bind both immune glycoproteins and microbial polysaccharide components, accommodating SIGN-R1’s ability to relate the recognition of microbes to the activation of the classical complement pathway.