Catalytic and structural role of a conserved active site histidine in berberine bridge enzyme.
Wallner, S., Winkler, A., Riedl, S., Dully, C., Horvath, S., Gruber, K., Macheroux, P.(2012) Biochemistry 51: 6139-6147
- PubMed: 22757961
- DOI: https://doi.org/10.1021/bi300411n
- Primary Citation of Related Structures:
4EC3 - PubMed Abstract:
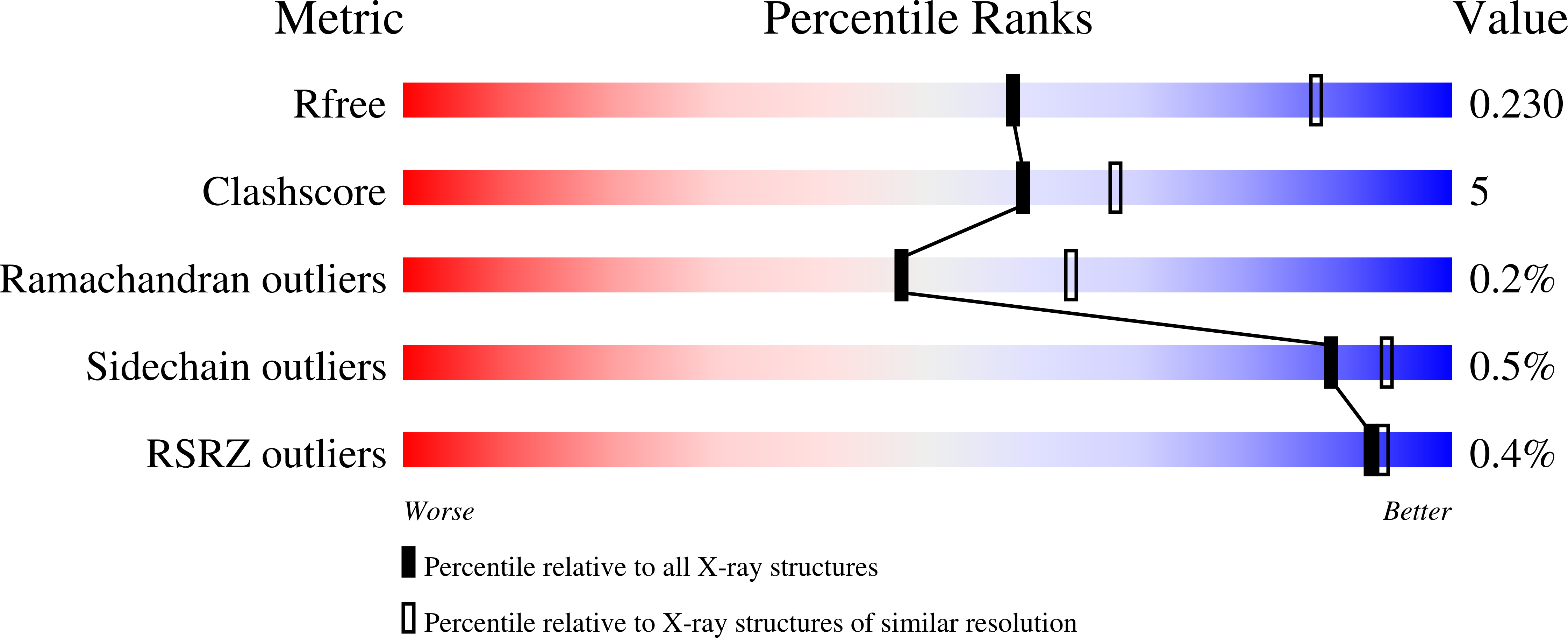
Berberine bridge enzyme (BBE) is a paradigm for the class of bicovalently flavinylated oxidases, which catalyzes the oxidative cyclization of (S)-reticuline to (S)-scoulerine. His174 was identified as an important active site residue because of its role in the stabilization of the reduced state of the flavin cofactor. It is also strictly conserved in the family of BBE-like oxidases. Here, we present a detailed biochemical and structural characterization of a His174Ala variant supporting its importance during catalysis and for the structural organization of the active site. Substantial changes in all kinetic parameters and a decrease in midpoint potential were observed for the BBE His174Ala variant protein. Moreover, the crystal structure of the BBE His174Ala variant showed significant structural rearrangements compared to wild-type enzyme. On the basis of our findings, we propose that His174 is part of a hydrogen bonding network that stabilizes the negative charge at the N1-C2=O locus via interaction with the hydroxyl group at C2' of the ribityl side chain of the flavin cofactor. Hence, replacement of this residue with alanine reduces the stabilizing effect for the transiently formed negative charge and results in drastically decreased kinetic parameters as well as a lower midpoint redox potential.
Organizational Affiliation:
Institute of Biochemistry, Graz University of Technology, Petersgasse 12/2, A-8010 Graz, Austria.