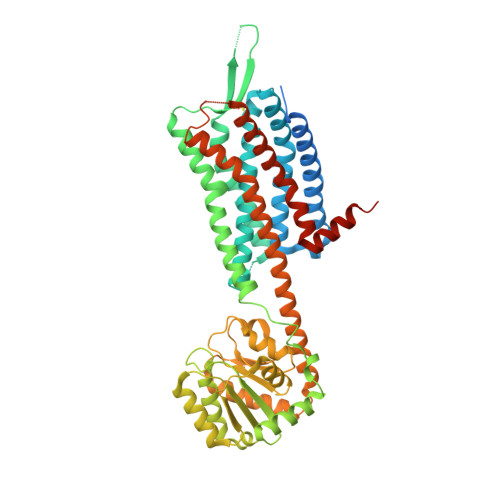
Crystal structure of the human OX2 orexin receptor bound to the insomnia drug suvorexant.
Yin, J., Mobarec, J.C., Kolb, P., Rosenbaum, D.M.(2015) Nature 519: 247-250
- PubMed: 25533960
- DOI: https://doi.org/10.1038/nature14035
- Primary Citation of Related Structures:
4S0V - PubMed Abstract:
The orexin (also known as hypocretin) G protein-coupled receptors (GPCRs) respond to orexin neuropeptides in the central nervous system to regulate sleep and other behavioural functions in humans. Defects in orexin signalling are responsible for the human diseases of narcolepsy and cataplexy; inhibition of orexin receptors is an effective therapy for insomnia. The human OX2 receptor (OX2R) belongs to the β branch of the rhodopsin family of GPCRs, and can bind to diverse compounds including the native agonist peptides orexin-A and orexin-B and the potent therapeutic inhibitor suvorexant. Here, using lipid-mediated crystallization and protein engineering with a novel fusion chimaera, we solved the structure of the human OX2R bound to suvorexant at 2.5 Å resolution. The structure reveals how suvorexant adopts a π-stacked horseshoe-like conformation and binds to the receptor deep in the orthosteric pocket, stabilizing a network of extracellular salt bridges and blocking transmembrane helix motions necessary for activation. Computational docking suggests how other classes of synthetic antagonists may interact with the receptor at a similar position in an analogous π-stacked fashion. Elucidation of the molecular architecture of the human OX2R expands our understanding of peptidergic GPCR ligand recognition and will aid further efforts to modulate orexin signalling for therapeutic ends.
Organizational Affiliation:
Department of Biophysics, The University of Texas Southwestern Medical Center, Dallas, Texas 75390, USA.