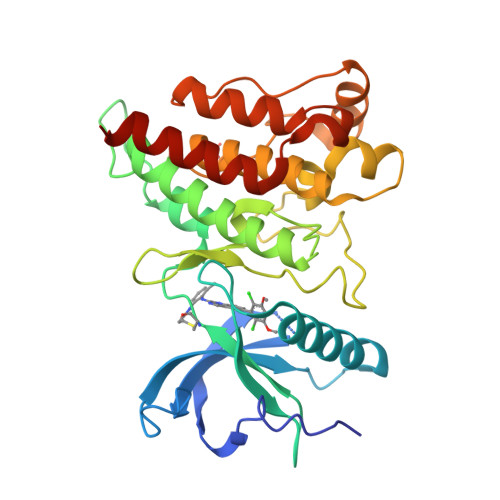
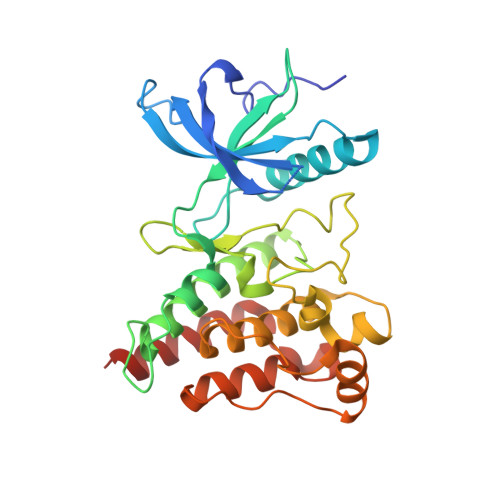
First Selective Small Molecule Inhibitor of FGFR4 for the Treatment of Hepatocellular Carcinomas with an Activated FGFR4 Signaling Pathway.
Hagel, M., Miduturu, C., Sheets, M., Rubin, N., Weng, W., Stransky, N., Bifulco, N., Kim, J.L., Hodous, B., Brooijmans, N., Shutes, A., Winter, C., Lengauer, C., Kohl, N.E., Guzi, T.(2015) Cancer Discov 5: 424-437
- PubMed: 25776529
- DOI: https://doi.org/10.1158/2159-8290.CD-14-1029
- Primary Citation of Related Structures:
4XCU - PubMed Abstract:
Aberrant signaling through the fibroblast growth factor 19 (FGF19)/fibroblast growth factor receptor 4 (FGFR 4) signaling complex has been shown to cause hepatocellular carcinoma (HCC) in mice and has been implicated to play a similar role in humans. We have developed BLU9931, a potent and irreversible small-molecule inhibitor of FGFR4, as a targeted therapy to treat patients with HCC whose tumors have an activated FGFR4 signaling pathway. BLU9931 is exquisitely selective for FGFR4 versus other FGFR family members and all other kinases. BLU9931 shows remarkable antitumor activity in mice bearing an HCC tumor xenograft that overexpresses FGF19 due to amplification as well as a liver tumor xenograft that overexpresses FGF19 mRNA but lacks FGF19 amplification. Approximately one third of patients with HCC whose tumors express FGF19 together with FGFR4 and its coreceptor klotho β (KLB) could potentially respond to treatment with an FGFR4 inhibitor. These findings are the first demonstration of a therapeutic strategy that targets a subset of patients with HCC. This article documents the discovery of BLU9931, a novel irreversible kinase inhibitor that specifically targets FGFR4 while sparing all other FGFR paralogs and demonstrates exquisite kinome selectivity. BLU9931 is efficacious in tumors with an intact FGFR4 signaling pathway that includes FGF19, FGFR4, and KLB. BLU9931 is the first FGFR4-selective molecule for the treatment of patients with HCC with aberrant FGFR4 signaling.
Organizational Affiliation:
Blueprint Medicines, Cambridge, Massachusetts.