X-ray Structural and Functional Studies of the Three Tandemly Linked Domains of Non-structural Protein 3 (nsp3) from Murine Hepatitis Virus Reveal Conserved Functions.
Chen, Y., Savinov, S.N., Mielech, A.M., Cao, T., Baker, S.C., Mesecar, A.D.(2015) J Biological Chem 290: 25293-25306
- PubMed: 26296883
- DOI: https://doi.org/10.1074/jbc.M115.662130
- Primary Citation of Related Structures:
4YPT - PubMed Abstract:
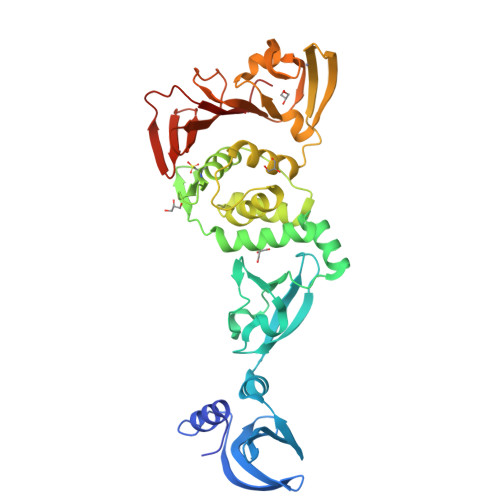
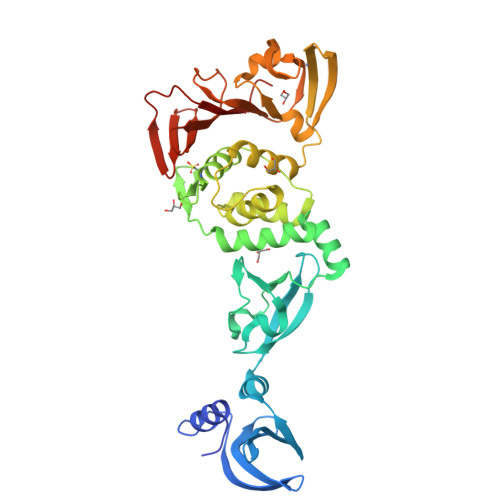
Murine hepatitis virus (MHV) has long served as a model system for the study of coronaviruses. Non-structural protein 3 (nsp3) is the largest nsp in the coronavirus genome, and it contains multiple functional domains that are required for coronavirus replication. Despite the numerous functional studies on MHV and its nsp3 domain, the structure of only one domain in nsp3, the small ubiquitin-like domain 1 (Ubl1), has been determined. We report here the x-ray structure of three tandemly linked domains of MHV nsp3, including the papain-like protease 2 (PLP2) catalytic domain, the ubiquitin-like domain 2 (Ubl2), and a third domain that we call the DPUP (domain preceding Ubl2 and PLP2) domain. DPUP has close structural similarity to the severe acute respiratory syndrome coronavirus unique domain C (SUD-C), suggesting that this domain may not be unique to the severe acute respiratory syndrome coronavirus. The PLP2 catalytic domain was found to have both deubiquitinating and deISGylating isopeptidase activities in addition to proteolytic activity. A computationally derived model of MHV PLP2 bound to ubiquitin was generated, and the potential interactions between ubiquitin and PLP2 were probed by site-directed mutagenesis. These studies extend substantially our structural knowledge of MHV nsp3, providing a platform for further investigation of the role of nsp3 domains in MHV viral replication.
Organizational Affiliation:
From the Department of Biological Sciences.