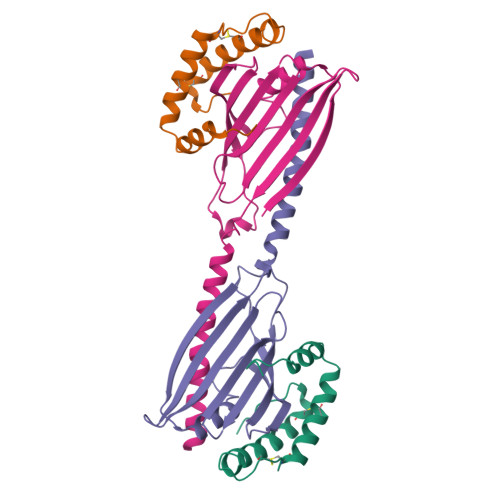
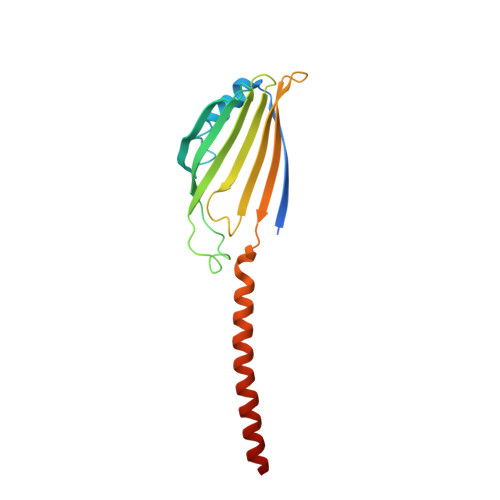
Structural and mechanistic insights into phospholipid transfer by Ups1-Mdm35 in mitochondria.
Watanabe, Y., Tamura, Y., Kawano, S., Endo, T.(2015) Nat Commun 6: 7922-7922
- PubMed: 26235513
- DOI: https://doi.org/10.1038/ncomms8922
- Primary Citation of Related Structures:
4YTV, 4YTW, 4YTX - PubMed Abstract:
Eukaryotic cells are compartmentalized into membrane-bounded organelles whose functions rely on lipid trafficking to achieve membrane-specific compositions of lipids. Here we focused on the Ups1-Mdm35 system, which mediates phosphatidic acid (PA) transfer between the outer and inner mitochondrial membranes, and determined the X-ray structures of Mdm35 and Ups1-Mdm35 with and without PA. The Ups1-Mdm35 complex constitutes a single domain that has a deep pocket and flexible Ω-loop lid. Structure-based mutational analyses revealed that a basic residue at the pocket bottom and the Ω-loop lid are important for PA extraction from the membrane following Ups1 binding. Ups1 binding to the membrane is enhanced by the dissociation of Mdm35. We also show that basic residues around the pocket entrance are important for Ups1 binding to the membrane and PA extraction. These results provide a structural basis for understanding the mechanism of PA transfer between mitochondrial membranes.
Organizational Affiliation:
1] Faculty of Life Sciences, Kyoto Sangyo University, Kamigamo-motoyama, Kita-ku, Kyoto, 603-8555, Japan [2] JST/CREST, Faculty of Life Sciences, Kyoto Sangyo University, Kamigamo-motoyama, Kita-ku, Kyoto, 603-8555, Japan [3] JST/CREST, Research Center for Materials Science, Nagoya University, Chikusa-ku, Nagoya, 464-8602, Japan.