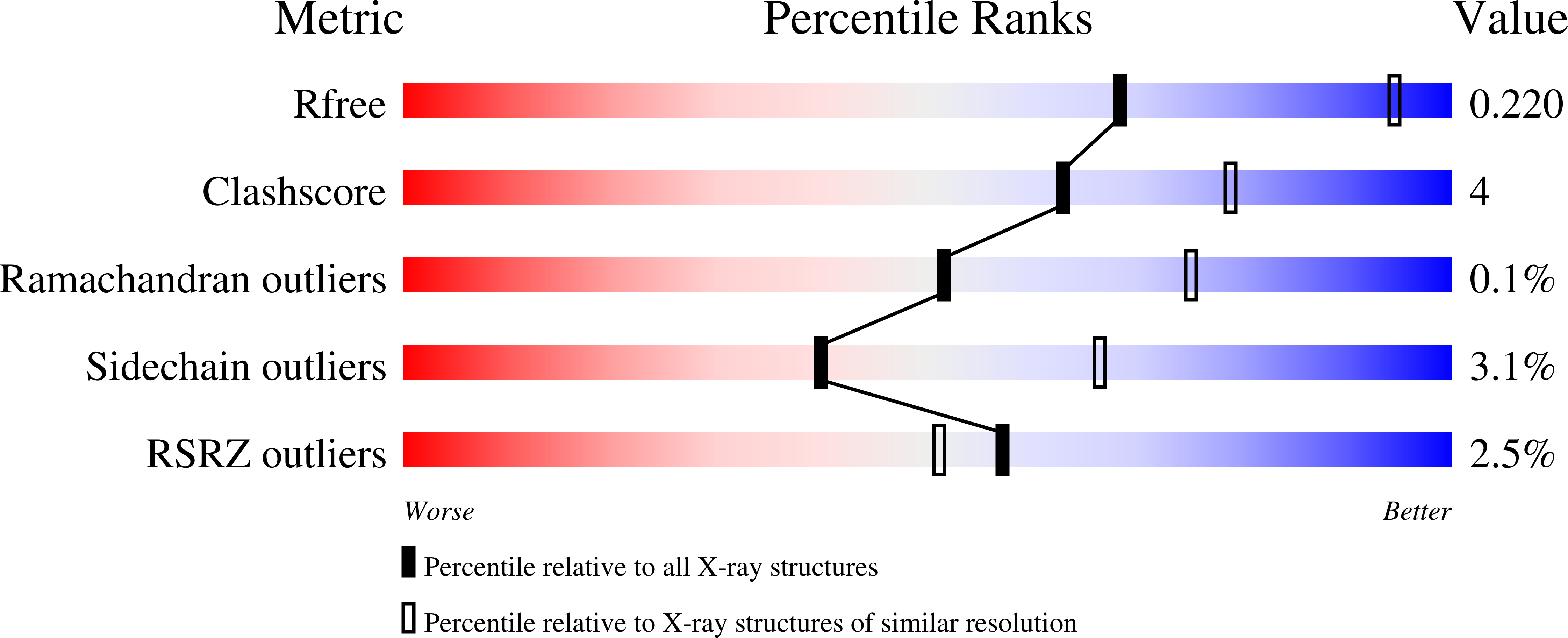
Unraveling the substrate recognition mechanism and specificity of the unusual glycosyl hydrolase family 29 BT2192 from Bacteroides thetaiotaomicron.
Guillotin, L., Lafite, P., Daniellou, R.(2014) Biochemistry 53: 1447-1455
- PubMed: 24527659
- DOI: https://doi.org/10.1021/bi400951q
- Primary Citation of Related Structures:
4OUE, 4OZO - PubMed Abstract:
Glycosyl hydrolase (GH) family 29 (CAZy database) consists of retaining α-l-fucosidases. We have identified BT2192, a protein from Bacteroides thetaiotaomicron, as the first GH29 representative exhibiting both weak α-l-fucosidase and β-d-galactosidase activities. Determination and analysis of X-ray structures of BT2192 in complex with β-d-galactoside competitive inhibitors showed a new binding mode different from that of known GH29 enzymes. Three point mutations, specific to BT2192, prevent the canonical GH29 substrate α-l-fucose from binding efficiently to the fucosidase-like active site relative to other GH29 enzymes. β-d-Galactoside analogues bind and interact in a second pocket, which is not visible in other reported GH29 structures. Molecular simulations helped in the assessment of the flexibility of both substrates in their respective pocket. Hydrolysis of the fucosyl moiety from the putative natural substrates like 3-fucosyllactose or Lewis(X) antigen would be mainly due to the efficient interactions with the galactosyl moiety, in the second binding site, located more than 6-7 Å apart.
Organizational Affiliation:
Université Orléans, CNRS, ICOA, UMR 7311 , F-45067 Orleans, France.