Discovery and structure-based optimization of adenain inhibitors.
Mac Sweeney, A., Grosche, P., Ellis, D., Combrink, K., Erbel, P., Hughes, N., Sirockin, F., Melkko, S., Bernardi, A., Ramage, P., Jarousse, N., Altmann, E.(2014) ACS Med Chem Lett 5: 937-941
- PubMed: 25147618
- DOI: https://doi.org/10.1021/ml500224t
- Primary Citation of Related Structures:
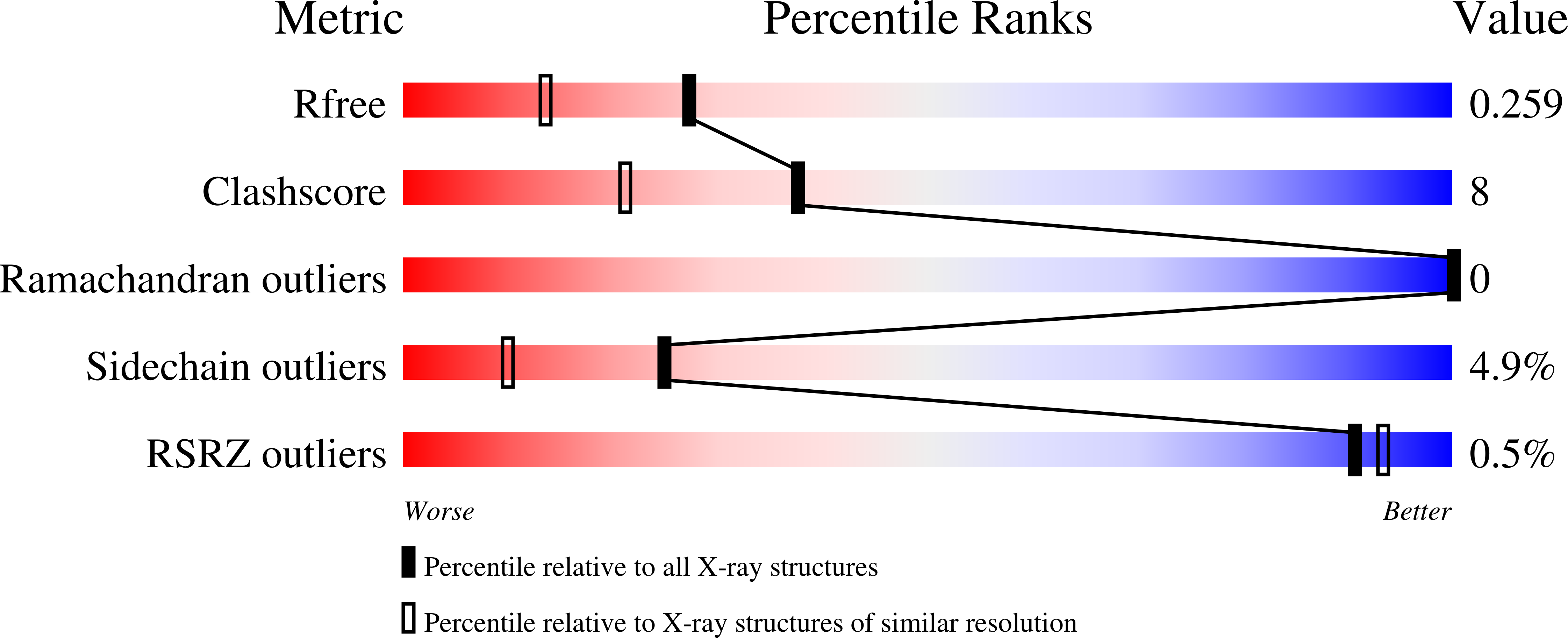
4PID, 4PIE, 4PIQ, 4PIS - PubMed Abstract:
The cysteine protease adenain is the essential protease of adenovirus and, as such, represents a promising target for the treatment of ocular and other adenoviral infections. Through a concise two-pronged hit discovery approach we identified tetrapeptide nitrile 1 and pyrimidine nitrile 2 as complementary starting points for adenain inhibition. These hits enabled the first high-resolution X-ray cocrystal structures of adenain with inhibitors bound and revealed the binding mode of 1 and 2. The screening hits were optimized by a structure-guided medicinal chemistry strategy into low nanomolar drug-like inhibitors of adenain.
Organizational Affiliation:
Novartis Institute for Biomedical Research , Novartis Campus, CH-4002 Basel, Switzerland.