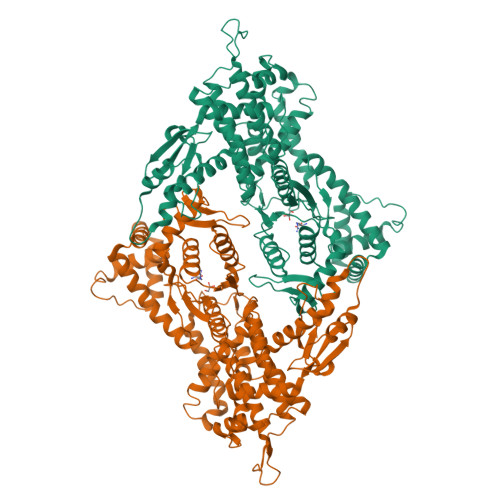
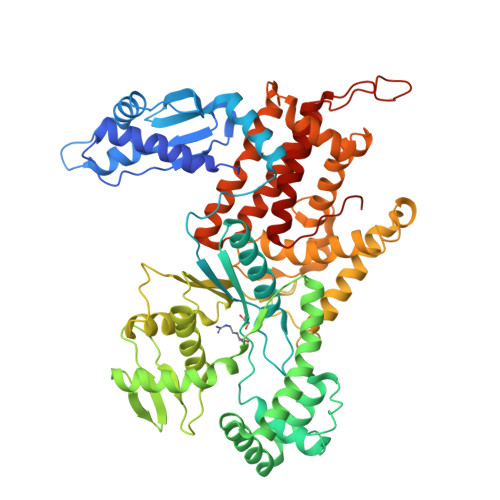
The crystal structure of arginyl-tRNA synthetase from Homo sapiens
Kim, H.S., Cha, S.Y., Jo, C.H., Han, A.R., Hwang, K.Y.(2014) FEBS Lett 588: 2328-2334
- PubMed: 24859084
- DOI: https://doi.org/10.1016/j.febslet.2014.05.027
- Primary Citation of Related Structures:
4Q2T, 4Q2X, 4Q2Y - PubMed Abstract:
Arginyl-tRNA synthetase (ArgRS) is a tRNA-binding protein that catalyzes the esterification of L-arginine to its cognate tRNA. L-Canavanine, a structural analog of L-arginine, has recently been studied as an anticancer agent. Here, we determined the crystal structures of the apo, L-arginine-complexed, and L-canavanine-complexed forms of the cytoplasmic free isoform of human ArgRS (hArgRS). Similar interactions were formed upon binding to L-canavanine or L-arginine, but the interaction between Tyr312 and the oxygen of the oxyguanidino group was a little bit different. Detailed conformational changes that occur upon substrate binding were explained. The hArgRS structure was also compared with previously reported homologue structures. The results presented here may provide a basis for the design of new anticancer drugs, such as L-canavanine analogs.
Organizational Affiliation:
Division of Biotechnology, College of Life Sciences and Biotechnology, Korea University, Seoul, Republic of Korea.