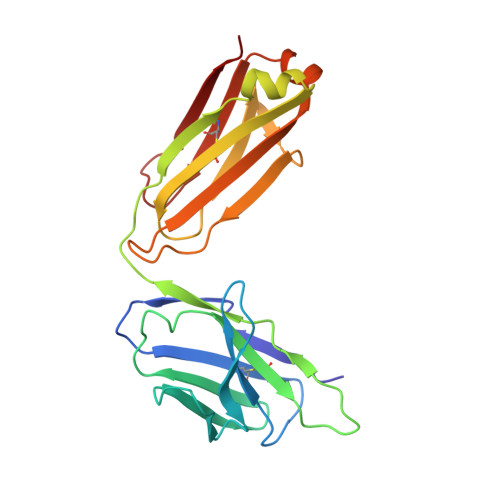
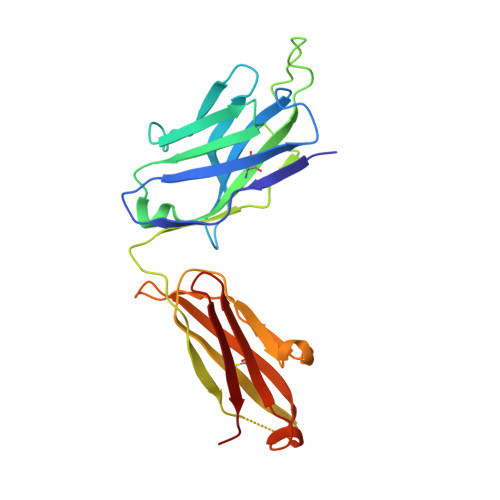
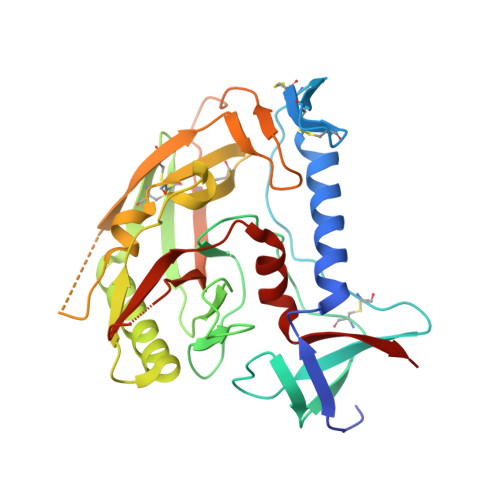
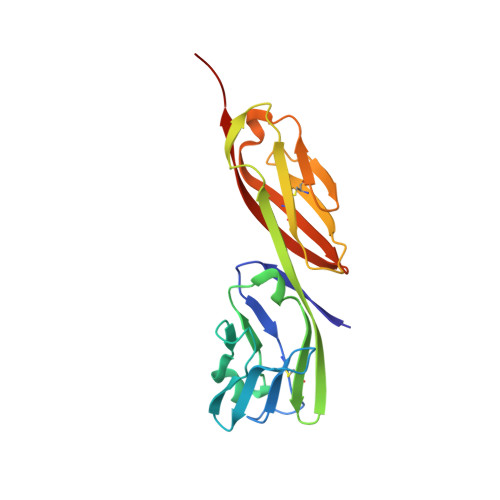Crystal structure of a fully glycosylated HIV-1 gp120 core reveals a stabilizing role for the glycan at Asn262.
Kong, L., Wilson, I.A., Kwong, P.D.(2015) Proteins 83: 590-596
- PubMed: 25546301
- DOI: https://doi.org/10.1002/prot.24747
- Primary Citation of Related Structures:
4RQS - PubMed Abstract:
The crystal structure of a fully glycosylated HIV-1 gp120 core in complex with CD4 receptor and Fab 17b at 4.5-Å resolution reveals 9 of the 15 N-linked glycans of core gp120 to be partially ordered. The glycan at position Asn262 had the most extensive and well-ordered electron density, and a GlcNAc(2)Man(7) was modeled. The GlcNAc stem of this glycan is largely buried in a cleft in gp120, suggesting a role in gp120 folding and stability. Its arms interact with the stems of neighboring glycans from the oligomannose patch, which is a major target for broadly neutralizing antibodies.
Organizational Affiliation:
Vaccine Research Center, National Institutes of Health, Bethesda, Maryland, 20892; Department of Integrative Structural and Computational Biology, the Scripps Research Institute, La Jolla, California; International AIDS Vaccine Initiative Neutralizing Antibody Center and the Collaboration for AIDS Vaccine Discovery, the Scripps Research Institute, La Jolla, California; Scripps Center for HIV/AIDS Vaccine Immunology and Immunogen Discovery, the Scripps Research Institute, La Jolla, California.