Potent nonimmunosuppressive cyclophilin inhibitors with improved pharmaceutical properties and decreased transporter inhibition.
Fu, J., Tjandra, M., Becker, C., Bednarczyk, D., Capparelli, M., Elling, R., Hanna, I., Fujimoto, R., Furegati, M., Karur, S., Kasprzyk, T., Knapp, M., Leung, K., Li, X., Lu, P., Mergo, W., Miault, C., Ng, S., Parker, D., Peng, Y., Roggo, S., Rivkin, A., Simmons, R.L., Wang, M., Wiedmann, B., Weiss, A.H., Xiao, L., Xie, L., Xu, W., Yifru, A., Yang, S., Zhou, B., Sweeney, Z.K.(2014) J Med Chem 57: 8503-8516
- PubMed: 25310383
- DOI: https://doi.org/10.1021/jm500862r
- Primary Citation of Related Structures:
4TOT - PubMed Abstract:
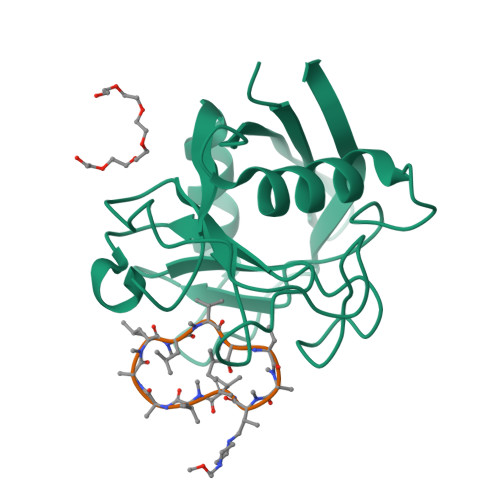
Nonimmunosuppressive cyclophilin inhibitors have demonstrated efficacy for the treatment of hepatitis C infection (HCV). However, alisporivir, cyclosporin A, and most other cyclosporins are potent inhibitors of OATP1B1, MRP2, MDR1, and other important drug transporters. Reduction of the side chain hydrophobicity of the P4 residue preserves cyclophilin binding and antiviral potency while decreasing transporter inhibition. Representative inhibitor 33 (NIM258) is a less potent transporter inhibitor relative to previously described cyclosporins, retains anti-HCV activity in cell culture, and has an acceptable pharmacokinetic profile in rats and dogs. An X-ray structure of 33 bound to rat cyclophilin D is reported.
Organizational Affiliation:
Novartis Institutes for Biomedical Research , 4560 Horton Street, Emeryville, California 94608, United States.