Modulation of ABA Signaling by Altering VxG Phi L Motif of PP2Cs in Oryza sativa.
Han, S., Min, M.K., Lee, S.Y., Lim, C.W., Bhatnagar, N., Lee, Y., Shin, D., Chung, K.Y., Lee, S.C., Kim, B.G., Lee, S.(2017) Mol Plant 10: 1190-1205
- PubMed: 28827170
- DOI: https://doi.org/10.1016/j.molp.2017.08.003
- Primary Citation of Related Structures:
5GWO, 5GWP - PubMed Abstract:
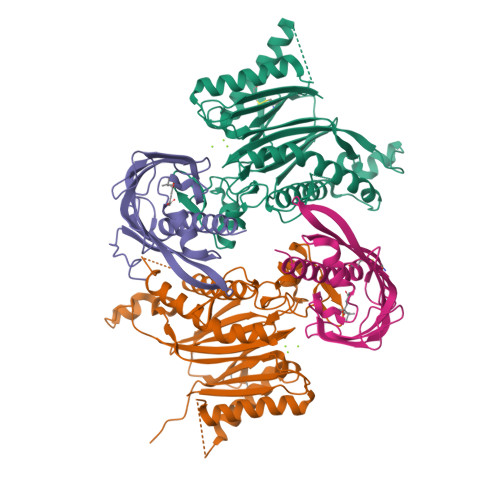
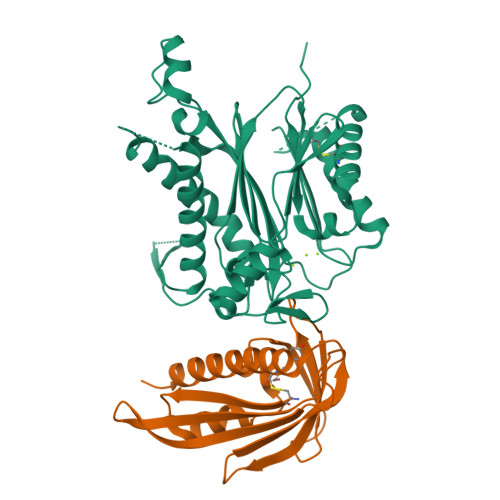
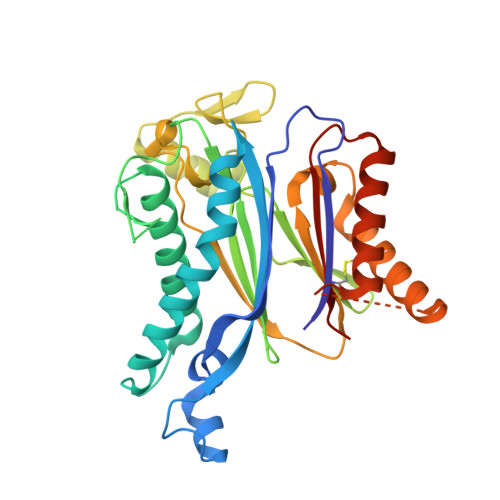
The abscisic acid (ABA) signaling pathway is regulated by clade A type 2C protein phosphatases (PP2CAs) in plants. In the presence of ABA, PP2Cs release stress/ABA-activated protein kinases by binding to ABA-bound receptors (PYL/RCARs) for activation. Although the wedging tryptophan in PP2Cs is critical in the interaction with PYL/RCARs in Arabidopsis and rice, it remains elusive as to how other interface regions are involved in the interaction. Here, we report the identification of a conserved region on PP2Cs, termed the VxGΦL motif, which modulates the interaction with PYL/RCARs through its second and fourth residues. The effects of the second and fourth residues on the interaction of OsPP2C50 with several OsPYL/RCAR proteins were investigated by systematic mutagenesis. One OsPP2C50 mutant, VFGML ("FM") mutant, lowered the affinity to OsPYL/RCAR3 by ∼15-fold in comparison with the wild-type. Comparison of the crystal structures of wild-type OsPP2C50:ABA:OsPYL/RCAR3 with those composed of FM mutant revealed local conformational changes near the VxGΦL motif, further supported by hydrogen-deuterium exchange mass spectrometry. In rice protoplasts, ABA signaling was altered by mutations in the VxGΦL motif. Transgenic Arabidopsis plants overexpressing OsPP2C50 and OsPP2C50FM showed altered ABA sensitivity. Taken together, the VxGΦL motif of PP2Cs appears to modulate the affinity of PP2Cs with PYL/RCARs and thus likely to alter the ABA signaling, leading to the differential sensitivity to ABA in planta.
Organizational Affiliation:
Department of Biological Sciences, Sungkyunkwan University, Suwon 16419, Republic of Korea.