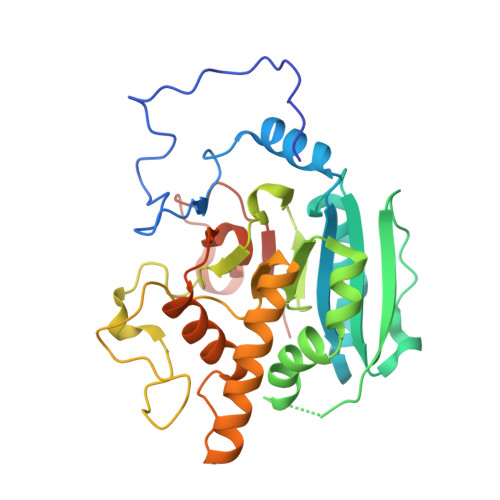
High Resolution Structures of the Human ABO(H) Blood Group Enzymes in Complex with Donor Analogs Reveal That the Enzymes Utilize Multiple Donor Conformations to Bind Substrates in a Stepwise Manner.
Gagnon, S.M., Meloncelli, P.J., Zheng, R.B., Haji-Ghassemi, O., Johal, A.R., Borisova, S.N., Lowary, T.L., Evans, S.V.(2015) J Biol Chem 290: 27040-27052
- PubMed: 26374898
- DOI: https://doi.org/10.1074/jbc.M115.682401
- Primary Citation of Related Structures:
5BXC, 5C1G, 5C1H, 5C1L, 5C36, 5C38, 5C3A, 5C3B, 5C3D, 5C47, 5C48, 5C49, 5C4B, 5C4C, 5C4D, 5C4E, 5C4F, 5C8R - PubMed Abstract:
Homologous glycosyltransferases α-(1→3)-N-acetylgalactosaminyltransferase (GTA) and α-(1→3)-galactosyltransferase (GTB) catalyze the final step in ABO(H) blood group A and B antigen synthesis through sugar transfer from activated donor to the H antigen acceptor. These enzymes have a GT-A fold type with characteristic mobile polypeptide loops that cover the active site upon substrate binding and, despite intense investigation, many aspects of substrate specificity and catalysis remain unclear. The structures of GTA, GTB, and their chimeras have been determined to between 1.55 and 1.39 Å resolution in complex with natural donors UDP-Gal, UDP-Glc and, in an attempt to overcome one of the common problems associated with three-dimensional studies, the non-hydrolyzable donor analog UDP-phosphono-galactose (UDP-C-Gal). Whereas the uracil moieties of the donors are observed to maintain a constant location, the sugar moieties lie in four distinct conformations, varying from extended to the "tucked under" conformation associated with catalysis, each stabilized by different hydrogen bonding partners with the enzyme. Further, several structures show clear evidence that the donor sugar is disordered over two of the observed conformations and so provide evidence for stepwise insertion into the active site. Although the natural donors can both assume the tucked under conformation in complex with enzyme, UDP-C-Gal cannot. Whereas UDP-C-Gal was designed to be "isosteric" with natural donor, the small differences in structure imposed by changing the epimeric oxygen atom to carbon appear to render the enzyme incapable of binding the analog in the active conformation and so preclude its use as a substrate mimic in GTA and GTB.
Organizational Affiliation:
Department of Biochemistry and Microbiology, University of Victoria, Victoria, British Columbia V8W 3P6, Canada and.