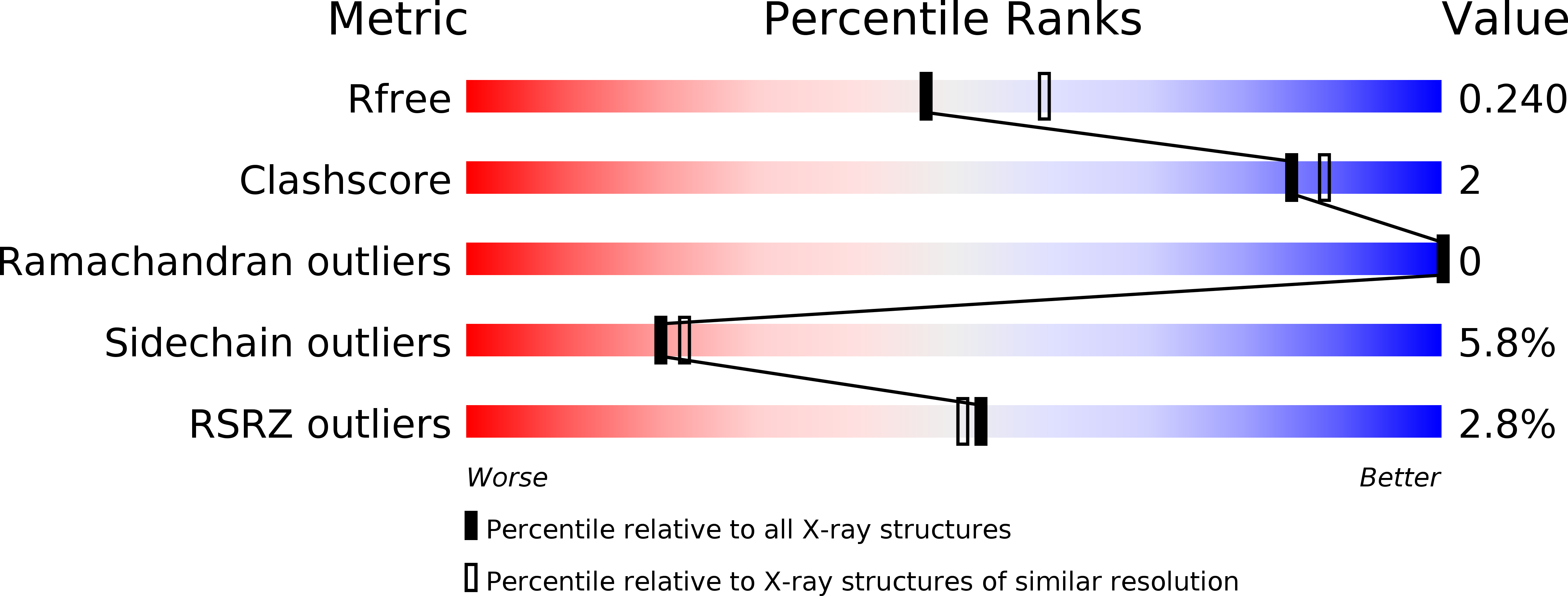
Potent Inhibitors of Acetyltransferase Eis Overcome Kanamycin Resistance in Mycobacterium tuberculosis.
Willby, M.J., Green, K.D., Gajadeera, C.S., Hou, C., Tsodikov, O.V., Posey, J.E., Garneau-Tsodikova, S.(2016) ACS Chem Biol 11: 1639-1646
- PubMed: 27010218
- DOI: https://doi.org/10.1021/acschembio.6b00110
- Primary Citation of Related Structures:
5EBV, 5EC4 - PubMed Abstract:
A major cause of tuberculosis (TB) resistance to the aminoglycoside kanamycin (KAN) is the Mycobacterium tuberculosis (Mtb) acetyltransferase Eis. Upregulation of this enzyme is responsible for inactivation of KAN through acetylation of its amino groups. A 123 000-compound high-throughput screen (HTS) yielded several small-molecule Eis inhibitors that share an isothiazole S,S-dioxide heterocyclic core. These were investigated for their structure-activity relationships. Crystal structures of Eis in complex with two potent inhibitors show that these molecules are bound in the conformationally adaptable aminoglycoside binding site of the enzyme, thereby obstructing binding of KAN for acetylation. Importantly, we demonstrate that several Eis inhibitors, when used in combination with KAN against resistant Mtb, efficiently overcome KAN resistance. This approach paves the way toward development of novel combination therapies against aminoglycoside-resistant TB.
Organizational Affiliation:
Division of Tuberculosis Elimination, National Center for HIV/AIDS, Viral Hepatitis, STD, and TB Prevention, Centers for Disease Control and Prevention , Atlanta, Georgia 30329, United States.