The Use of Fluoroproline in MUC1 Antigen Enables Efficient Detection of Antibodies in Patients with Prostate Cancer.
Somovilla, V.J., Bermejo, I.A., Albuquerque, I.S., Martinez-Saez, N., Castro-Lopez, J., Garcia-Martin, F., Companon, I., Hinou, H., Nishimura, S.I., Jimenez-Barbero, J., Asensio, J.L., Avenoza, A., Busto, J.H., Hurtado-Guerrero, R., Peregrina, J.M., Bernardes, G.J.L., Corzana, F.(2017) J Am Chem Soc 139: 18255-18261
- PubMed: 29166012
- DOI: https://doi.org/10.1021/jacs.7b09447
- Primary Citation of Related Structures:
5OWP - PubMed Abstract:
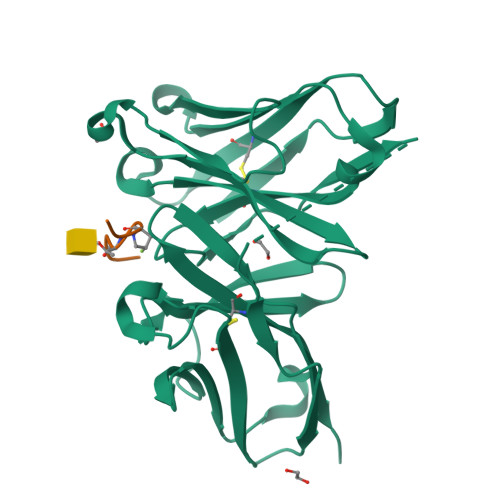
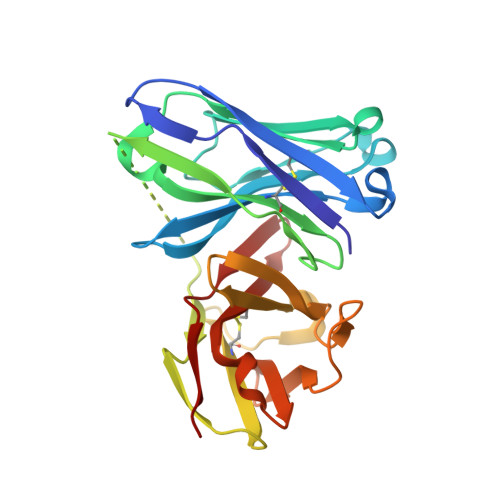
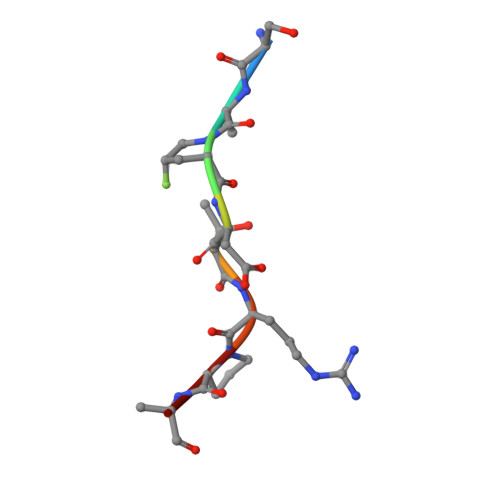
A structure-based design of a new generation of tumor-associated glycopeptides with improved affinity against two anti-MUC1 antibodies is described. These unique antigens feature a fluorinated proline residue, such as a (4S)-4-fluoro-l-proline or 4,4-difluoro-l-proline, at the most immunogenic domain. Binding assays using biolayer interferometry reveal 3-fold to 10-fold affinity improvement with respect to the natural (glyco)peptides. According to X-ray crystallography and MD simulations, the fluorinated residues stabilize the antigen-antibody complex by enhancing key CH/π interactions. Interestingly, a notable improvement in detection of cancer-associated anti-MUC1 antibodies from serum of patients with prostate cancer is achieved with the non-natural antigens, which proves that these derivatives can be considered better diagnostic tools than the natural antigen for prostate cancer.
Organizational Affiliation:
Departamento de Química, Universidad de La Rioja, Centro de Investigación en Síntesis Química , 26006 Logroño, Spain.