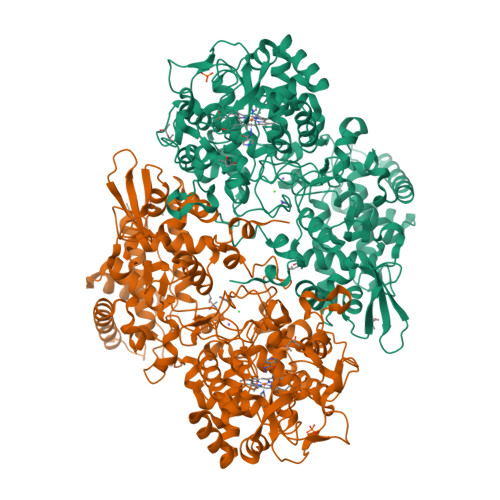
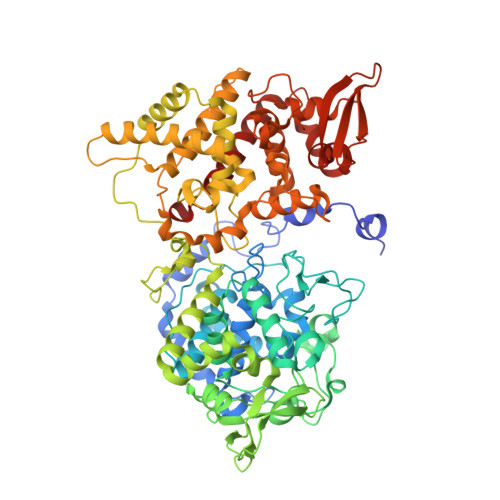
Binding of the antitubercular pro-drug isoniazid in the heme access channel of catalase-peroxidase (KatG). A combined structural and metadynamics investigation.
Vidossich, P., Loewen, P.C., Carpena, X., Fiorin, G., Fita, I., Rovira, C.(2014) J Phys Chem B 118: 2924-2931
- PubMed: 24568093
- DOI: https://doi.org/10.1021/jp4123425
- Primary Citation of Related Structures:
5SYI, 5SYJ - PubMed Abstract:
Isonicotinic acid hydrazide (isoniazid or INH) is a front line antitubercular pro-drug that is converted to its active form, isonicotinyl-NAD, by the bacterial catalase-peroxidase KatG. Understanding the role of KatG in the INH activation process has been hampered by a lack of knowledge of the actual drug binding site. In this work, we have investigated the binding of INH in the main access channel of KatG with a combination of molecular dynamics, using an enhanced-sampling technique (metadynamics), X-ray crystallography, and site-directed mutagenesis. The metadynamics simulations show that there are several weak drug binding sites along the access channel. Moreover, the simulations evidence that complete entrance to the heme active site is impeded by an aspartate residue (D141) located above the heme. This has been confirmed by structural and functional analysis of the D141A mutant, leading to the first X-ray crystallography evidence of INH at the heme access channel.
Organizational Affiliation:
Unitat de Química Física, Departament de Química, Universitat Autònoma de Barcelona , 08193 Bellaterra, Spain.