Structure-Based Design of a Soluble Prefusion-Closed HIV-1 Env Trimer with Reduced CD4 Affinity and Improved Immunogenicity.
Chuang, G.Y., Geng, H., Pancera, M., Xu, K., Cheng, C., Acharya, P., Chambers, M., Druz, A., Tsybovsky, Y., Wanninger, T.G., Yang, Y., Doria-Rose, N.A., Georgiev, I.S., Gorman, J., Joyce, M.G., O'Dell, S., Zhou, T., McDermott, A.B., Mascola, J.R., Kwong, P.D.(2017) J Virol 91
- PubMed: 28275193
- DOI: https://doi.org/10.1128/JVI.02268-16
- Primary Citation of Related Structures:
5UTF, 5UTY - PubMed Abstract:
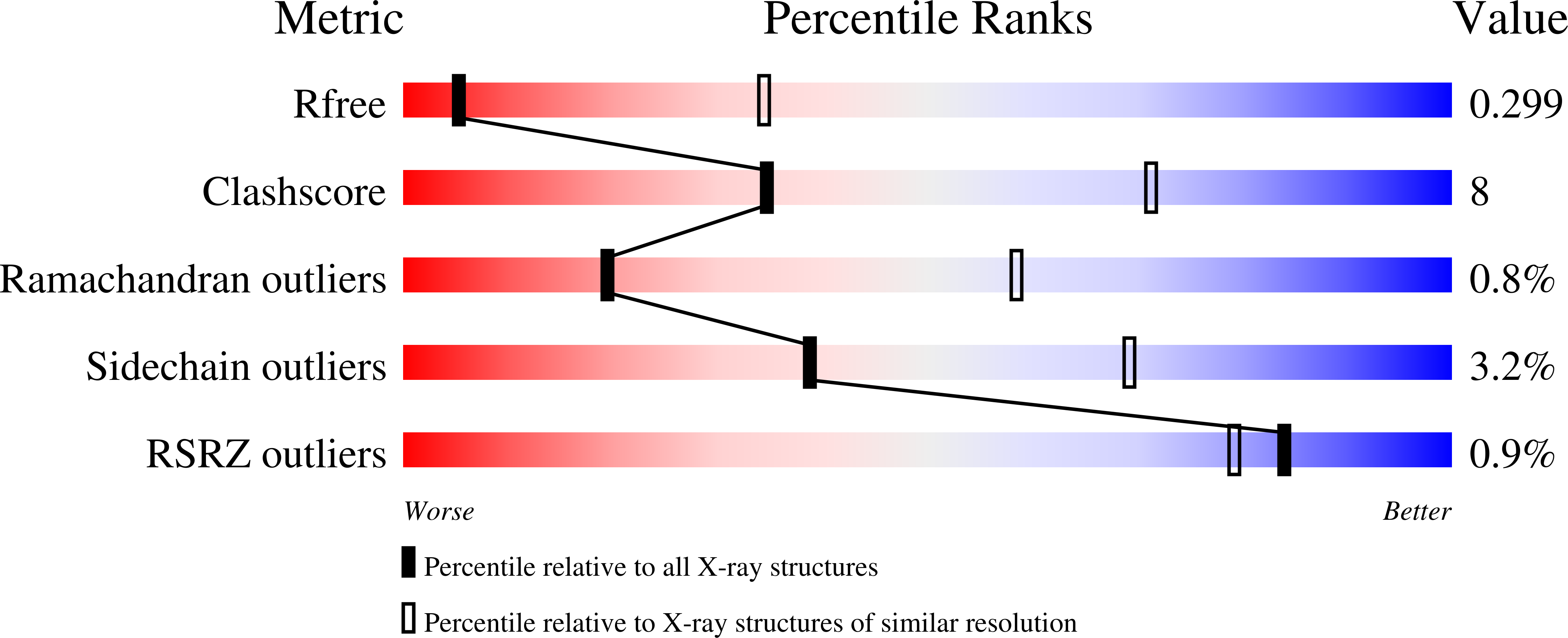
The HIV-1 envelope (Env) trimer is a target for vaccine design as well as a conformational machine that facilitates virus entry by transitioning between prefusion-closed, CD4-bound, and coreceptor-bound conformations by transitioning into a postfusion state. Vaccine designers have sought to restrict the conformation of the HIV-1 Env trimer to its prefusion-closed state as this state is recognized by most broadly neutralizing, but not nonneutralizing, antibodies. We previously identified a disulfide bond, I201C-A433C (DS), which stabilizes Env in the vaccine-desired prefusion-closed state. When placed into the context of BG505 SOSIP.664, a soluble Env trimer mimic developed by Sanders, Moore, and colleagues, the engineered DS-SOSIP trimer showed reduced conformational triggering by CD4. Here, we further stabilize DS-SOSIP through a combination of structure-based design and 96-well-based expression and antigenic assessment. From 103 designs, we identified one, named DS-SOSIP.4mut, with four additional mutations at the interface of potentially mobile domains of the prefusion-closed structure. We also determined the crystal structures of DS-SOSIP.4mut at 4.1-Å resolution and of an additional DS-SOSIP.6mut variant at 4.3-Å resolution, and these confirmed the formation of engineered disulfide bonds. Notably, DS-SOSIP.4mut elicited a higher ratio of tier 2 autologous titers versus tier 1 V3-sensitive titers than BG505 SOSIP.664. DS-SOSIP.4mut also showed reduced recognition of CD4 and increased thermostability. The improved antigenicity, thermostability, and immunogenicity of DS-SOSIP.4mut suggest utility as an immunogen or a serologic probe; moreover, the specific four alterations identified here, M154, M300, M302, and L320 (4mut), can also be transferred to other HIV-1 Env trimers of interest to improve their properties. IMPORTANCE One approach to elicit broadly neutralizing antibodies against HIV-1 is to stabilize the structurally flexible HIV-1 envelope (Env) trimer in a conformation that displays predominantly broadly neutralizing epitopes and few to no nonneutralizing epitopes. The prefusion-closed conformation of HIV-1 Env has been identified as one such preferred conformation, and a current leading vaccine candidate is the BG505 DS-SOSIP variant, comprising two disulfides and an Ile-to-Pro mutation of Env from strain BG505. Here, we introduced additional mutations to further stabilize BG505 DS-SOSIP in the vaccine-preferred prefusion-closed conformation. In guinea pigs, our best mutant, DS-SOSIP.4mut, elicited a significantly higher ratio of autologous versus V3-directed neutralizing antibody responses than the SOSIP-stabilized form. We also observed an improvement in thermostability and a reduction in CD4 affinity. With improved antigenicity, stability, and immunogenicity, DS-SOSIP.4mut-stabilized trimers may have utility as HIV-1 immunogens or in other antigen-specific contexts, such as with B-cell probes.
Organizational Affiliation:
Vaccine Research Center, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, Maryland, USA.