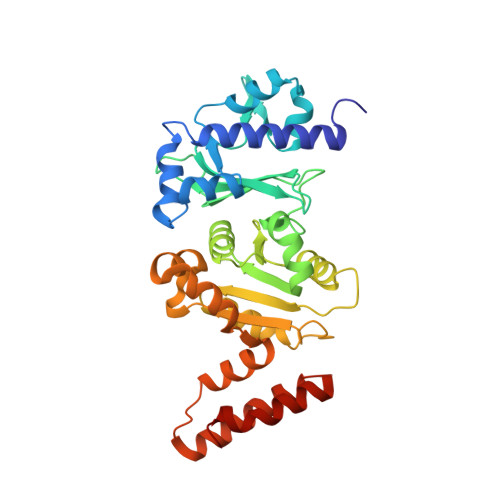
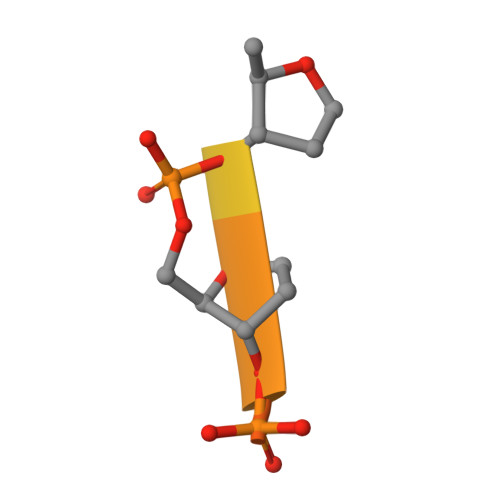
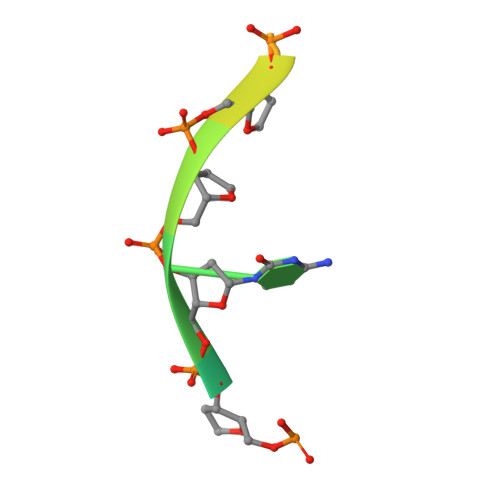
Structures of the Catalytic Domain of Bacterial Primase DnaG in Complexes with DNA Provide Insight into Key Priming Events.
Hou, C., Biswas, T., Tsodikov, O.V.(2018) Biochemistry 57: 2084-2093
- PubMed: 29558114
- DOI: https://doi.org/10.1021/acs.biochem.8b00036
- Primary Citation of Related Structures:
5W33, 5W34, 5W35, 5W36 - PubMed Abstract:
Bacterial primase DnaG is an essential nucleic acid polymerase that generates primers for replication of chromosomal DNA. The mechanism of DnaG remains unclear due to the paucity of structural information on DnaG in complexes with other replisome components. Here we report the first crystal structures of noncovalent DnaG-DNA complexes, obtained with the RNA polymerase domain of Mycobacterium tuberculosis DnaG and various DNA ligands. One structure, obtained with ds DNA, reveals interactions with DnaG as it slides on ds DNA and suggests how DnaG binds template for primer synthesis. In another structure, DNA in the active site of DnaG mimics the primer, providing insight into mechanisms for the nucleotide transfer and DNA translocation. In conjunction with the recent cryo-EM structure of the bacteriophage T7 replisome, this study yields a model for primer elongation and hand-off to DNA polymerase.
Organizational Affiliation:
Department of Pharmaceutical Sciences, College of Pharmacy , University of Kentucky , Lexington , Kentucky 40536 , United States.