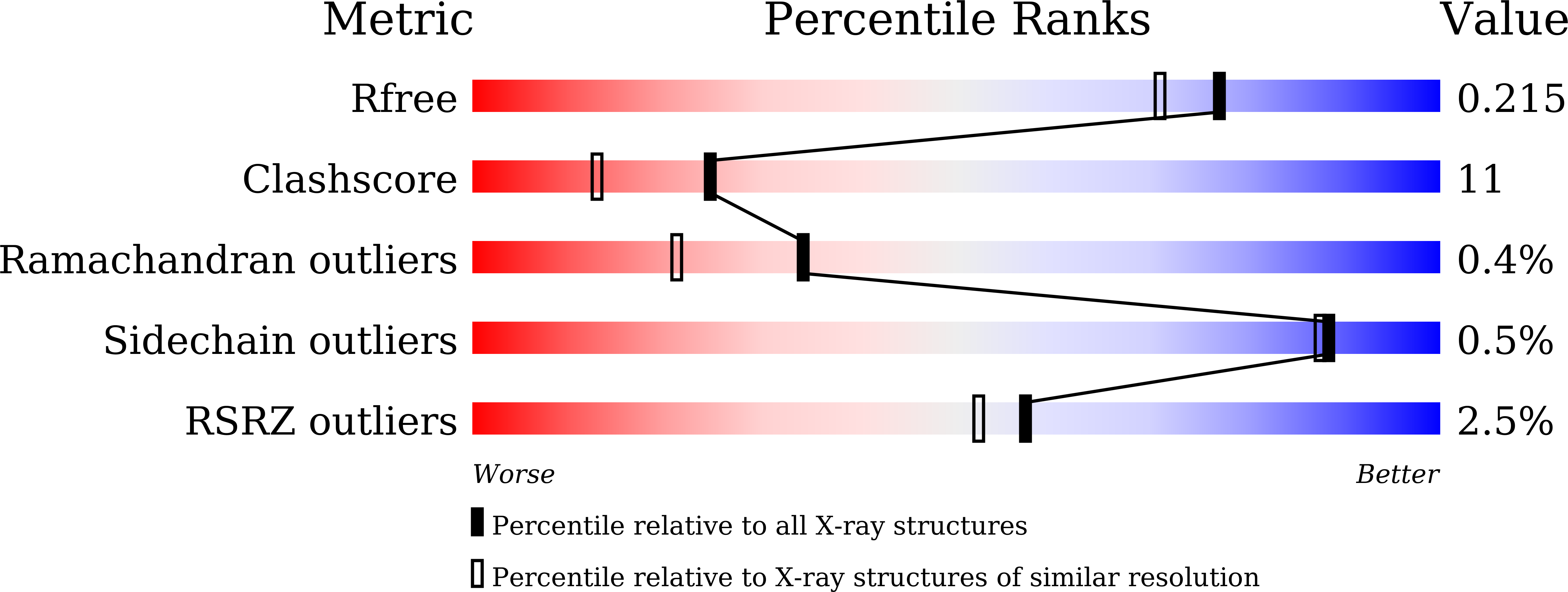
Glutathione transferase Omega 1-1 (GSTO1-1) modulates Akt and MEK1/2 signaling in human neuroblastoma cell SH-SY5Y.
Saisawang, C., Wongsantichon, J., Robinson, R.C., Ketterman, A.J.(2019) Proteins 87: 588-595
- PubMed: 30874320
- DOI: https://doi.org/10.1002/prot.25683
- Primary Citation of Related Structures:
5YVN, 5YVO - PubMed Abstract:
In the human neuroblastoma SH-SY5Y cell line, the glutathione transferase Omega 1-1 (GSTO1-1) appears to modulate Akt and MEK1/2 kinase activation. We observed a glutathionylation modification was involved in the activation of Akt but not MEK1/2. With the specific GSTO1-1 inhibitor ML175, we show the enzyme activity of GSTO1-1 is important for modulation as the inhibited GSTO1-1 allowed activation of both Akt and MEK1/2. The inhibition of GSTO1-1 showed a similar extent of activation of Akt and MEK1/2 as treatment by the endotoxin lipopolysaccharide. The GSTO1-1 also either directly interacts with Akt and MEK1/2 or interacts with a protein complexed with Akt and MEK1/2 as both kinases coimmunoprecipitated with GSTO1-1. The results suggest that GSTO1-1 enzyme activity inhibits the activation of these two kinases to maintain basal levels. The possible regulation by GSTO1-1 is of interest as both kinases have hundreds of potential downstream targets that are known to have contributions to various cellular processes including survival, growth, proliferation, and metabolism.
Organizational Affiliation:
Institute of Molecular Biosciences, Mahidol University, Salaya, Nakhon Pathom, Thailand.