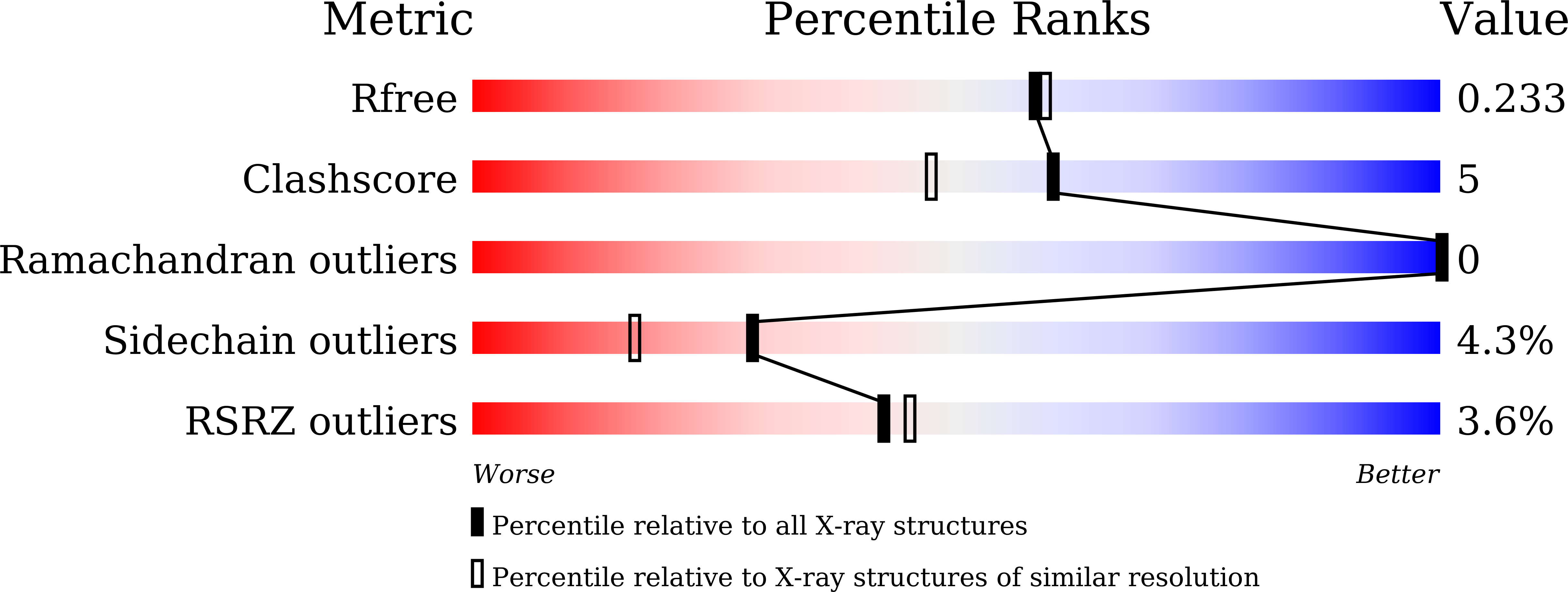
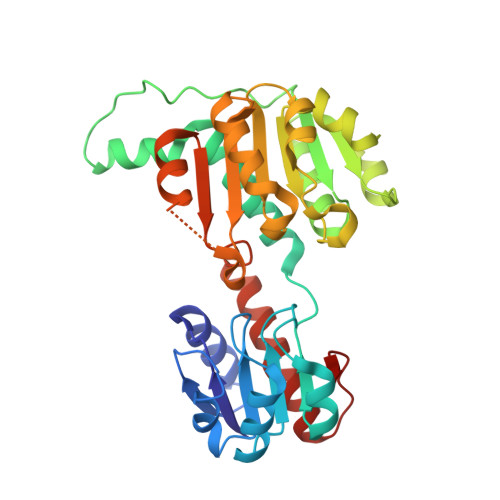
Structural Basis of Sequential Allosteric Transitions in Tetrameric d-Lactate Dehydrogenases from Three Gram-Negative Bacteria.
Furukawa, N., Miyanaga, A., Nakajima, M., Taguchi, H.(2018) Biochemistry 57: 5388-5406
- PubMed: 30149697
- DOI: https://doi.org/10.1021/acs.biochem.8b00557
- Primary Citation of Related Structures:
5Z1Z, 5Z20, 5Z21, 6ABI, 6ABJ - PubMed Abstract:
d-Lactate dehydrogenases (d-LDHs) from Fusobacterium nucleatum (FnLDH) and Escherichia coli (EcLDH) exhibit positive cooperativity in substrate binding, and the Pseudomonas aeruginosa enzyme (PaLDH) shows negatively cooperative substrate binding. The apo and ternary complex structures of FnLDH and PaLDH have been determined together with the apo-EcLDH structure. The three enzymes consistently form homotetrameric structures with three symmetric axes, the P-, Q-, and R-axes, unlike Lactobacillus d-LDHs, P-axis-related dimeric enzymes, although apo-FnLDH and EcLDH form asymmetric and distorted quaternary structures. The tetrameric structure allows apo-FnLDH and EcLDH to form wide intersubunit contact surfaces between the opened catalytic domains of the two Q-axis-related subunits in coordination with their asymmetric and distorted quaternary structures. These contact surfaces comprise intersubunit hydrogen bonds and hydrophobic interactions and likely prevent the domain closure motion during initial substrate binding. In contrast, apo-PaLDH possesses a highly symmetrical quaternary structure and partially closed catalytic domains that are favorable for initial substrate binding and forms virtually no intersubunit contact surface between the catalytic domains, which present their negatively charged surfaces to each other at the subunit interface. Complex FnLDH and PaLDH possess highly symmetrical quaternary structures with closed forms of the catalytic domains, which are separate from each other at the subunit interface. Structure-based mutations successfully converted the three enzymes to their dimeric forms, which exhibited no significant cooperativity in substrate binding. These observations indicate that the three enzymes undergo typical sequential allosteric transitions to exhibit their distinctive allosteric functions through the tetrameric structures.
Organizational Affiliation:
Department of Applied Biological Science, Faculty of Science and Technology , Tokyo University of Science , 2641 Yamazaki , Noda , Chiba 278-8510 , Japan.