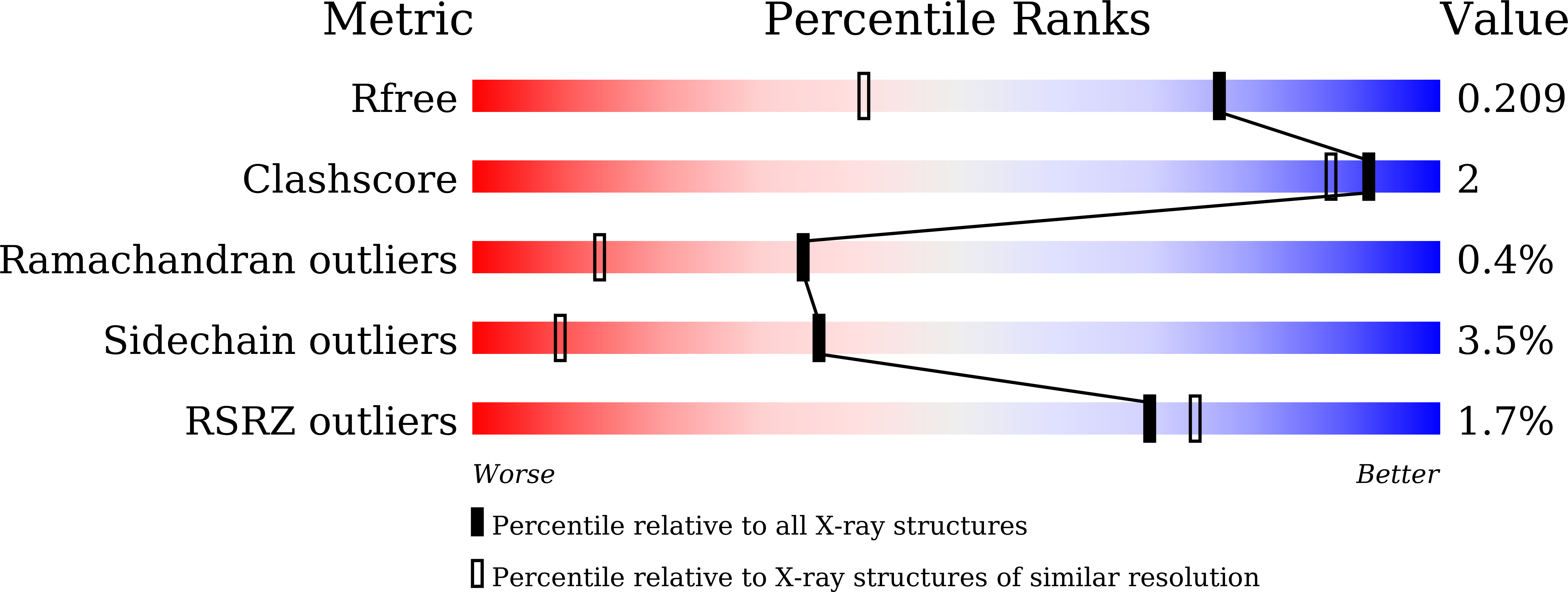
The hydrolytic water molecule of Class A beta-lactamase relies on the acyl-enzyme intermediate ES* for proper coordination and catalysis.
He, Y., Lei, J., Pan, X., Huang, X., Zhao, Y.(2020) Sci Rep 10: 10205-10205
- PubMed: 32576842
- DOI: https://doi.org/10.1038/s41598-020-66431-w
- Primary Citation of Related Structures:
5ZFL, 5ZFT, 5ZG6 - PubMed Abstract:
Serine-based β-lactamases of Class A, C and D all rely on a key water molecule to hydrolyze and inactivate β-lactam antibiotics. This process involves two conserved catalytic steps. In the first acylation step, the β-lactam antibiotic forms an acyl-enzyme intermediate (ES*) with the catalytic serine residue. In the second deacylation step, an activated water molecule serves as nucleophile (WAT_Nu) to attack ES* and release the inactivated β-lactam. The coordination and activation of WAT_Nu is not fully understood. Using time-resolved x-ray crystallography and QM/MM simulations, we analyzed three intermediate structures of Class A β-lactamase PenP as it slowly hydrolyzed cephaloridine. WAT_Nu is centrally located in the apo structure but becomes slightly displaced away by ES* in the post-acylation structure. In the deacylation structure, WAT_Nu moves back and is positioned along the Bürgi-Dunitz trajectory with favorable energetic profile to attack ES*. Unexpectedly, WAT_Nu is also found to adopt a catalytically incompetent conformation in the deacylation structure forming a hydrogen bond with ES*. Our results reveal that ES* plays a significant role in coordinating and activating WAT_Nu through subtle yet distinct interactions at different stages of the catalytic process. These interactions may serve as potential targets to circumvent β-lactamase-mediated antibiotic resistance.
Organizational Affiliation:
The Hong Kong Polytechnic University Shenzhen Research Institute, Shenzhen, P. R. China.