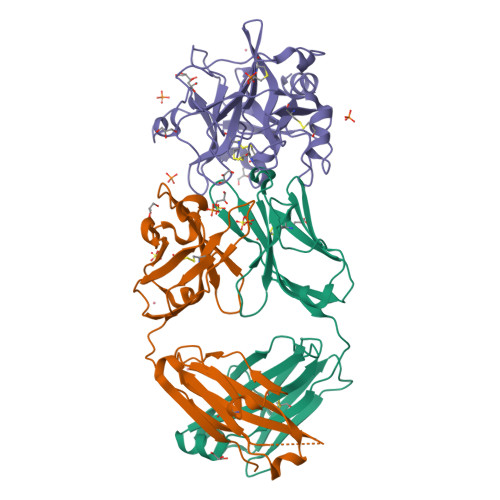
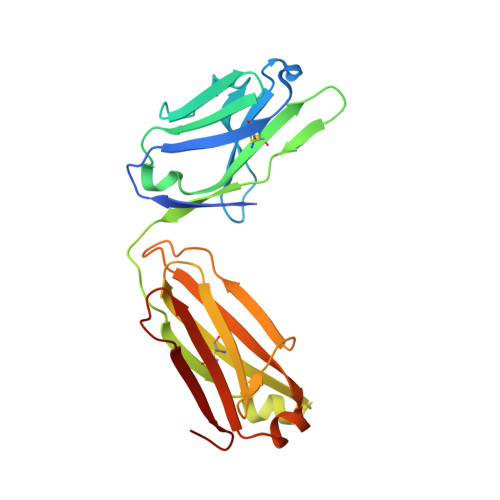
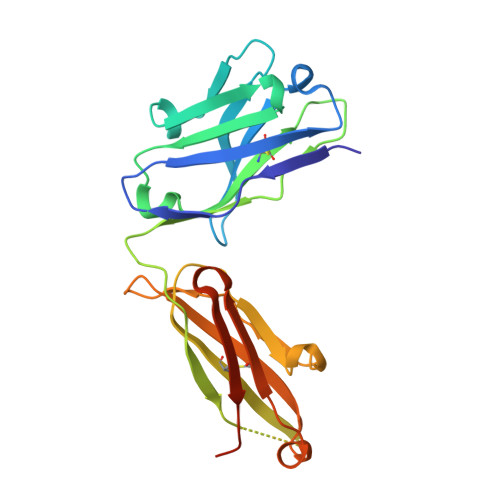
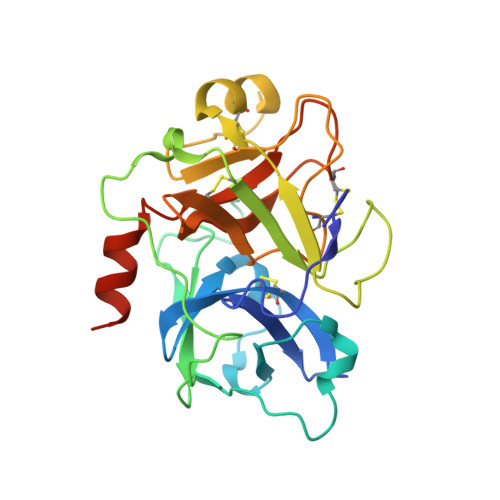
Structural Basis for Activity and Specificity of an Anticoagulant Anti-FXIa Monoclonal Antibody and a Reversal Agent.
Ely, L.K., Lolicato, M., David, T., Lowe, K., Kim, Y.C., Samuel, D., Bessette, P., Garcia, J.L., Mikita, T., Minor, D.L., Coughlin, S.R.(2018) Structure 26: 187-198.e4
- PubMed: 29336885
- DOI: https://doi.org/10.1016/j.str.2017.12.010
- Primary Citation of Related Structures:
6AOD - PubMed Abstract:
Coagulation factor XIa is a candidate target for anticoagulants that better separate antithrombotic efficacy from bleeding risk. We report a co-crystal structure of the FXIa protease domain with DEF, a human monoclonal antibody that blocks FXIa function and prevents thrombosis in animal models without detectable increased bleeding. The light chain of DEF occludes the FXIa S1 subsite and active site, while the heavy chain provides electrostatic interactions with the surface of FXIa. The structure accounts for the specificity of DEF for FXIa over its zymogen and related proteases, its active-site-dependent binding, and its ability to inhibit substrate cleavage. The inactive FXIa protease domain used to obtain the DEF-FXIa crystal structure reversed anticoagulant activity of DEF in plasma and in vivo and the activity of a small-molecule FXIa active-site inhibitor in vitro. DEF and this reversal agent for FXIa active-site inhibitors may help support clinical development of FXIa-targeting anticoagulants.
Organizational Affiliation:
Centers for Therapeutic Innovation, San Francisco, Pfizer Inc., 1700 Owens Street, San Francisco, CA 94158, USA.