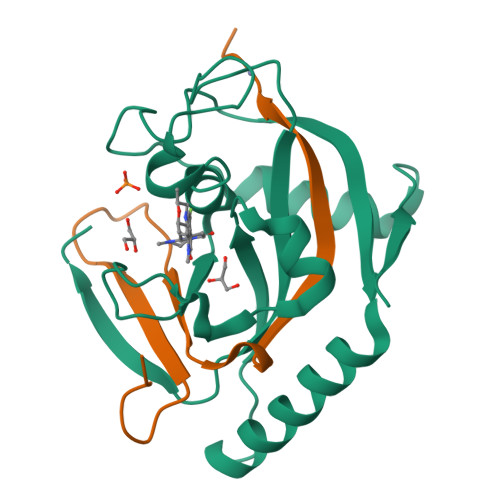
Design and Discovery of an Orally Efficacious Spiroindolinone-Based Tankyrase Inhibitor for the Treatment of Colon Cancer.
Shirai, F., Mizutani, A., Yashiroda, Y., Tsumura, T., Kano, Y., Muramatsu, Y., Chikada, T., Yuki, H., Niwa, H., Sato, S., Washizuka, K., Koda, Y., Mazaki, Y., Jang, M.K., Yoshida, H., Nagamori, A., Okue, M., Watanabe, T., Kitamura, K., Shitara, E., Honma, T., Umehara, T., Shirouzu, M., Fukami, T., Seimiya, H., Yoshida, M., Koyama, H.(2020) J Med Chem 63: 4183-4204
- PubMed: 32202790
- DOI: https://doi.org/10.1021/acs.jmedchem.0c00045
- Primary Citation of Related Structures:
6KRO - PubMed Abstract:
Tankyrases (TNKS/TNKS2) belong to the poly(ADP-ribose) polymerase family. Inhibition of their enzymatic activities attenuates the Wnt/β-catenin signaling, which plays an important role in cancer pathogenesis. We previously reported the discovery of RK-287107, a spiroindoline-based, highly selective, potent tankyrase inhibitor. Herein we describe the optimization process of RK-287107 leading to RK-582, which exhibits a markedly improved robust tumor growth inhibition in a COLO-320DM mouse xenograft model when orally administered. In addition to the dose-dependent elevation and attenuation of the levels of biomarkers AXIN2 and β-catenin, respectively, results of the TCF reporter and cell proliferation studies on COLO-320DM are discussed.
Organizational Affiliation:
Division of Molecular Biotherapy, Cancer Chemotherapy Center, Japanese Foundation for Cancer Research, 3-8-31 Ariake, Koto-ku, Tokyo 135-8850, Japan.