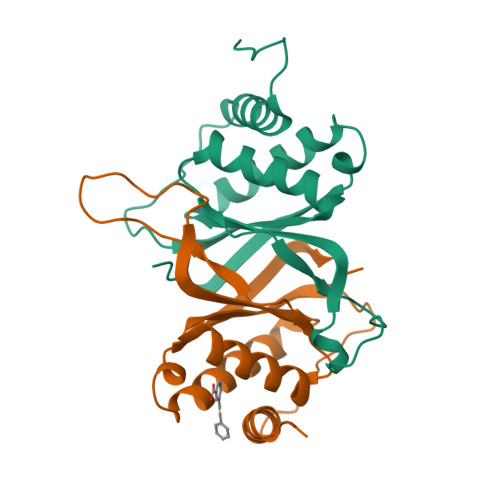
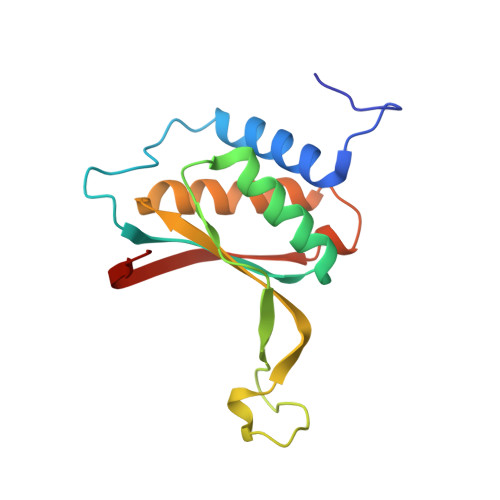
Structure-based design of small-molecule inhibitors of EBNA1 DNA binding blocks Epstein-Barr virus latent infection and tumor growth.
Messick, T.E., Smith, G.R., Soldan, S.S., McDonnell, M.E., Deakyne, J.S., Malecka, K.A., Tolvinski, L., van den Heuvel, A.P.J., Gu, B.W., Cassel, J.A., Tran, D.H., Wassermann, B.R., Zhang, Y., Velvadapu, V., Zartler, E.R., Busson, P., Reitz, A.B., Lieberman, P.M.(2019) Sci Transl Med 11
- PubMed: 30842315
- DOI: https://doi.org/10.1126/scitranslmed.aau5612
- Primary Citation of Related Structures:
6NPI, 6NPM, 6NPP - PubMed Abstract:
Epstein-Barr virus (EBV) is a DNA tumor virus responsible for 1 to 2% of human cancers including subtypes of Burkitt's lymphoma, Hodgkin's lymphoma, gastric carcinoma, and nasopharyngeal carcinoma (NPC). Persistent latent infection drives EBV-associated tumorigenesis. Epstein-Barr nuclear antigen 1 (EBNA1) is the only viral protein consistently expressed in all EBV-associated tumors and is therefore an attractive target for therapeutic intervention. It is a multifunctional DNA binding protein critical for viral replication, genome maintenance, viral gene expression, and host cell survival. Using a fragment-based approach and x-ray crystallography, we identify a 2,3-disubstituted benzoic acid series that selectively inhibits the DNA binding activity of EBNA1. We characterize these inhibitors biochemically and in cell-based assays, including chromatin immunoprecipitation and DNA replication assays. In addition, we demonstrate the potency of EBNA1 inhibitors to suppress tumor growth in several EBV-dependent xenograft models, including patient-derived xenografts for NPC. These inhibitors selectively block EBV gene transcription and alter the cellular transforming growth factor-β (TGF-β) signaling pathway in NPC tumor xenografts. These EBNA1-specific inhibitors show favorable pharmacological properties and have the potential to be further developed for the treatment of EBV-associated malignancies.
Organizational Affiliation:
The Wistar Institute, 3601 Spruce Street, Philadelphia, PA 19104, USA. lieberman@wistar.org troymessick@gmail.com.