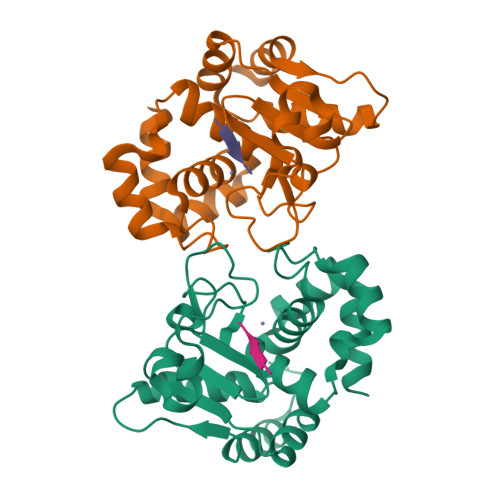
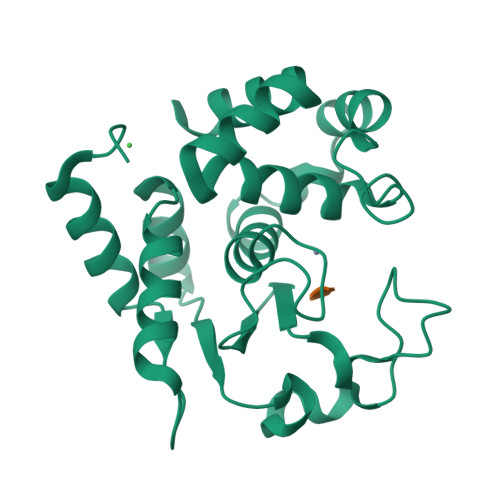
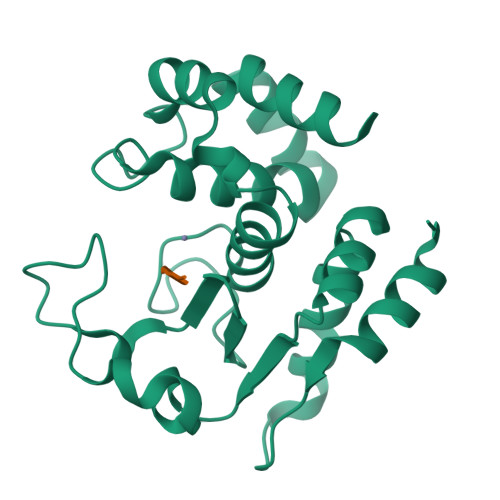
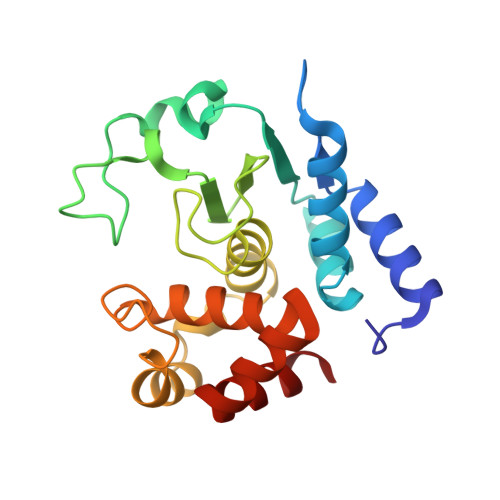
Molecular determinants of the mechanism and substrate specificity ofClostridium difficileproline-proline endopeptidase-1.
Pichlo, C., Juetten, L., Wojtalla, F., Schacherl, M., Diaz, D., Baumann, U.(2019) J Biological Chem 294: 11525-11535
- PubMed: 31182482
- DOI: https://doi.org/10.1074/jbc.RA119.009029
- Primary Citation of Related Structures:
6R4W, 6R4X, 6R4Y, 6R4Z, 6R50, 6R51, 6R52, 6R53, 6R54, 6R55, 6R56, 6R57, 6R58, 6R59, 6R5A, 6R5B, 6R5C, 6R9Z - PubMed Abstract:
Pro-Pro endopeptidase-1 (PPEP-1) is a secreted metalloprotease from the bacterial pathogen Clostridium difficile that cleaves two endogenous adhesion proteins. PPEP-1 is therefore important for bacterial motility and hence for efficient gut colonization during infection. PPEP-1 exhibits a unique specificity for Pro-Pro peptide bonds within the consensus sequence VNP↓PVP. In this study, we combined information from crystal and NMR structures with mutagenesis and enzyme kinetics to investigate the mechanism and substrate specificity of PPEP-1. Our analyses revealed that the substrate-binding cleft of PPEP-1 is shaped complementarily to the major conformation of the substrate in solution. We found that it possesses features that accept a tertiary amide and help discriminate P1' residues by their amide hydrogen bond-donating potential. We also noted that residues Lys-101, Trp-103, and Glu-184 are crucial for proteolytic activity. Upon substrate binding, these residues position a flexible loop over the substrate-binding cleft and modulate the second coordination sphere of the catalytic zinc ion. On the basis of these findings, we propose an induced-fit model in which prestructured substrates are recognized followed by substrate positioning within the active-site cleft and a concomitant increase in the Lewis acidity of the catalytic Zn 2+ ion. In conclusion, our findings provide detailed structural and mechanistic insights into the substrate recognition and specificity of PPEP-1 from the common gut pathogen C. difficile .
Organizational Affiliation:
Department of Chemistry, Institute of Biochemistry, University of Cologne, 50674 Cologne, Germany.