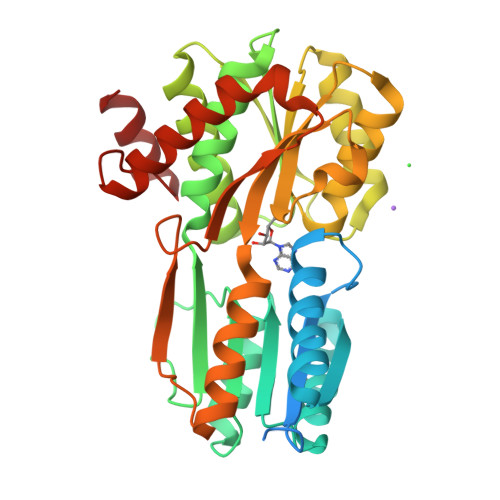
Structural and Biomolecular Analyses of Borrelia burgdorferi BmpD Reveal a Substrate-Binding Protein of an ABC-Type Nucleoside Transporter Family.
Cuellar, J., Astrand, M., Elovaara, H., Pietikainen, A., Siren, S., Liljeblad, A., Guedez, G., Salminen, T.A., Hytonen, J.(2020) Infect Immun 88
- PubMed: 31988175
- DOI: https://doi.org/10.1128/IAI.00962-19
- Primary Citation of Related Structures:
6SHU - PubMed Abstract:
Borrelia burgdorferi sensu lato , the causative agent of tick-borne Lyme borreliosis (LB), has a limited metabolic capacity and needs to acquire nutrients, such as amino acids, fatty acids, and nucleic acids, from the host environment. Using X-ray crystallography, liquid chromatography-mass spectrometry, microscale thermophoresis, and cellular localization studies, we show that basic membrane protein D (BmpD) is a periplasmic substrate-binding protein of an ABC transporter system binding to purine nucleosides. Nucleosides are essential for bacterial survival in the host organism, and these studies suggest a key role for BmpD in the purine salvage pathway of B. burgdorferi sensu lato Because B. burgdorferi sensu lato lacks the enzymes required for de novo purine synthesis, BmpD may play a vital role in ensuring access to the purines needed to sustain an infection in the host. Furthermore, we show that, although human LB patients develop anti-BmpD antibodies, immunization of mice with BmpD does not confer protection against B. burgdorferi sensu lato infection.
Organizational Affiliation:
Institute of Biomedicine, Faculty of Medicine, University of Turku, Turku, Finland julia.cuellar@utu.fi.