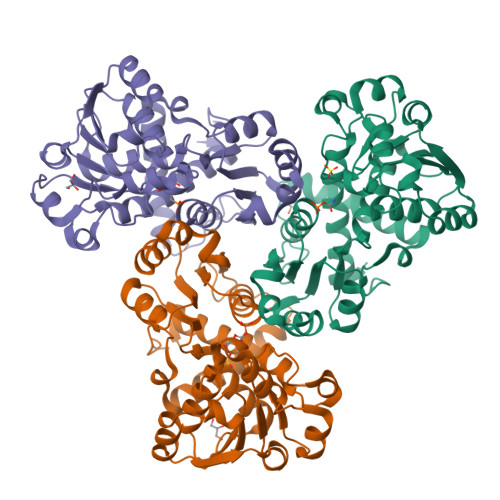
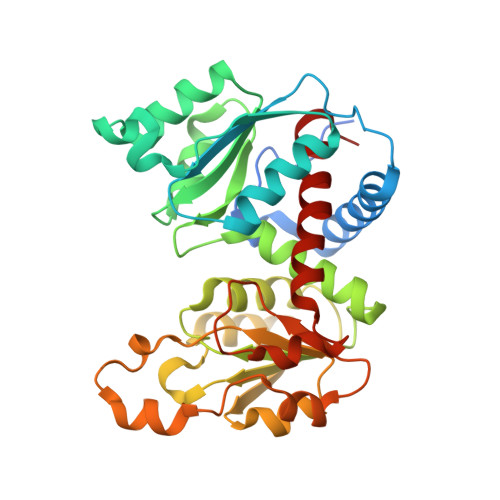
Mechanisms of feedback inhibition and sequential firing of active sites in plant aspartate transcarbamoylase.
Bellin, L., Del Cano-Ochoa, F., Velazquez-Campoy, A., Mohlmann, T., Ramon-Maiques, S.(2021) Nat Commun 12: 947-947
- PubMed: 33574254
- DOI: https://doi.org/10.1038/s41467-021-21165-9
- Primary Citation of Related Structures:
6YPO, 6YS6, 6YSP, 6YVB, 6YW9, 6YWJ, 6YY1 - PubMed Abstract:
Aspartate transcarbamoylase (ATC), an essential enzyme for de novo pyrimidine biosynthesis, is uniquely regulated in plants by feedback inhibition of uridine 5-monophosphate (UMP). Despite its importance in plant growth, the structure of this UMP-controlled ATC and the regulatory mechanism remain unknown. Here, we report the crystal structures of Arabidopsis ATC trimer free and bound to UMP, complexed to a transition-state analog or bearing a mutation that turns the enzyme insensitive to UMP. We found that UMP binds and blocks the ATC active site, directly competing with the binding of the substrates. We also prove that UMP recognition relies on a loop exclusively conserved in plants that is also responsible for the sequential firing of the active sites. In this work, we describe unique regulatory and catalytic properties of plant ATCs that could be exploited to modulate de novo pyrimidine synthesis and plant growth.
Organizational Affiliation:
Pflanzenphysiologie, Fachbereich Biologie, Universität Kaiserslautern, Erwin-Schrödinger-Strasse, D-67663, Kaiserslautern, Germany.