Targeting Tyrosinase: Development and Structural Insights of Novel Inhibitors Bearing Arylpiperidine and Arylpiperazine Fragments.
Ferro, S., Deri, B., Germano, M.P., Gitto, R., Ielo, L., Buemi, M.R., Certo, G., Vittorio, S., Rapisarda, A., Pazy, Y., Fishman, A., De Luca, L.(2018) J Med Chem 61: 3908-3917
- PubMed: 29634898
- DOI: https://doi.org/10.1021/acs.jmedchem.7b01745
- Primary Citation of Related Structures:
5OAE, 6EI4 - PubMed Abstract:
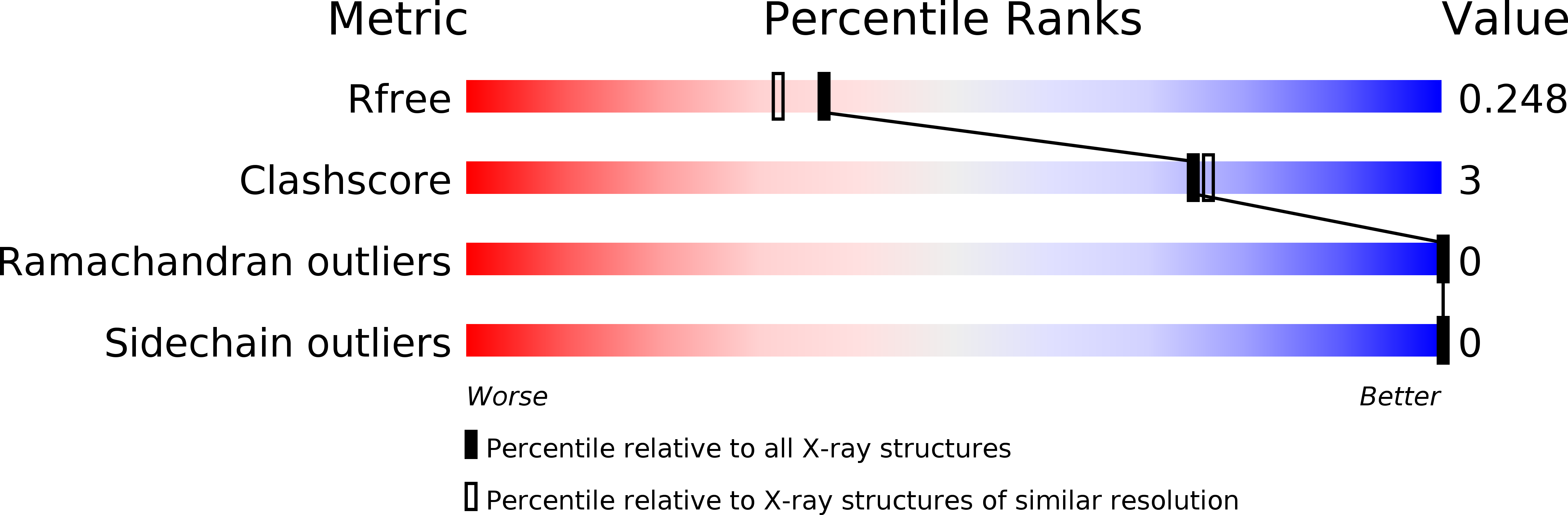
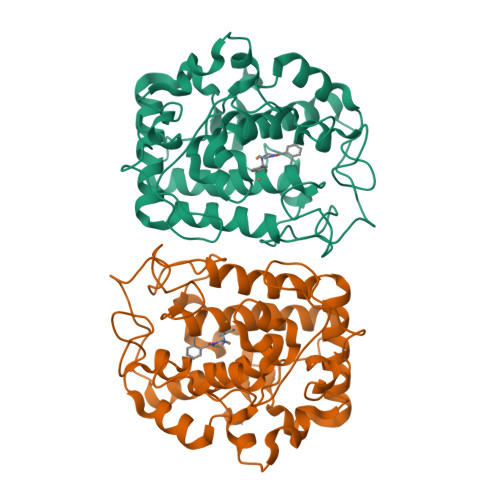
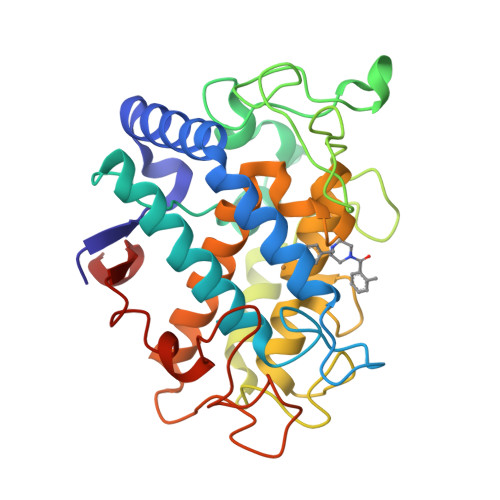
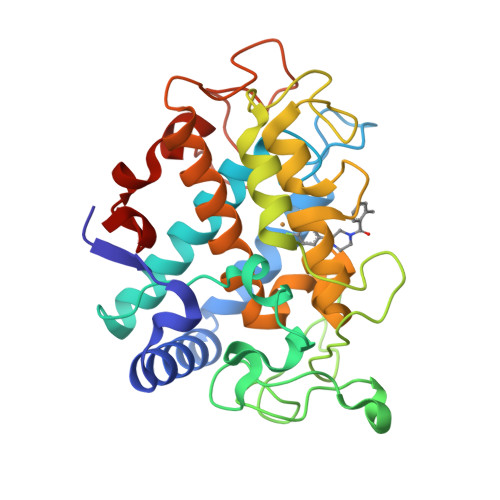
The inhibition of tyrosinase (Ty, EC 1.14.18.1) represents an efficient strategy of decreasing melanogenesis and skin hyperpigmentation. A combination of crystallographic and docking studies on two different tyrosinases, that from Bacillus megaterium (TyBm) and that from a mushroom (TyM), has contributed to increasing our knowledge about their structural information and translating that information to the most druggable human Ty (TyH) isozyme. In particular, we designed and synthesized a series of 1-(4-fluorobenzyl)piperazine and 1-(4-fluorobenzyl)piperidine derivatives showing inhibitory activities on TyM at micromolar ranges and more potency than that of the reference compound, kojic acid. The crystal structures of TyBm with inhibitor 3 (IC 50 value of 25.11 μM) and 16 (IC 50 value of 5.25 μM) were solved, confirming the binding poses hypothesized by in silico studies and revealing the main molecular determinants for the binding recognition of the inhibitors.
Organizational Affiliation:
Dipartimento di Scienze Chimiche, Biologiche, Farmaceutiche e Ambientali (CHIBIOFARAM) , Polo Universitario SS. Annunziata, Università di Messina , Viale Annunziata , I-98168 Messina , Italy.