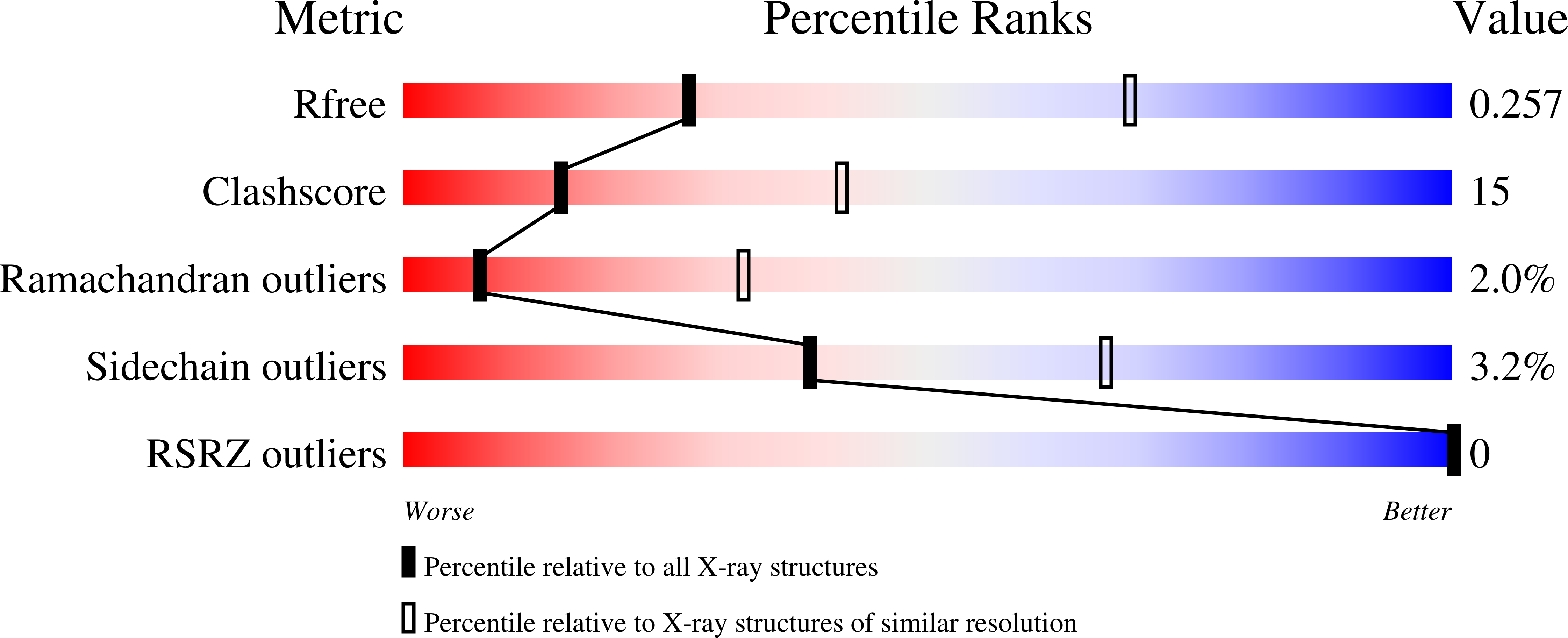
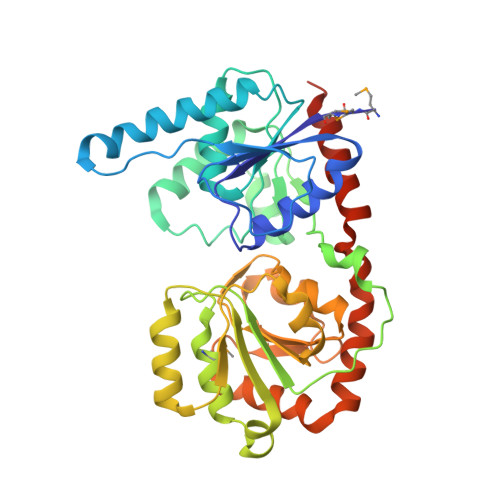
Structural basis of the molecular ruler mechanism of a bacterial glycosyltransferase.
Ramirez, A.S., Boilevin, J., Mehdipour, A.R., Hummer, G., Darbre, T., Reymond, J.L., Locher, K.P.(2018) Nat Commun 9: 445-445
- PubMed: 29386647
- DOI: https://doi.org/10.1038/s41467-018-02880-2
- Primary Citation of Related Structures:
6EJI, 6EJJ, 6EJK - PubMed Abstract:
The membrane-associated, processive and retaining glycosyltransferase PglH from Campylobacter jejuni is part of the biosynthetic pathway of the lipid-linked oligosaccharide (LLO) that serves as the glycan donor in bacterial protein N-glycosylation. Using an unknown counting mechanism, PglH catalyzes the transfer of exactly three α1,4 N-acetylgalactosamine (GalNAc) units to the growing LLO precursor, GalNAc-α1,4-GalNAc-α1,3-Bac-α1-PP-undecaprenyl. Here, we present crystal structures of PglH in three distinct states, including a binary complex with UDP-GalNAc and two ternary complexes containing a chemo-enzymatically generated LLO analog and either UDP or synthetic, nonhydrolyzable UDP-CH 2 -GalNAc. PglH contains an amphipathic helix ("ruler helix") that has a dual role of facilitating membrane attachment and glycan counting. The ruler helix contains three positively charged side chains that can bind the pyrophosphate group of the LLO substrate and thus limit the addition of GalNAc units to three. These results, combined with molecular dynamics simulations, provide the mechanism of glycan counting by PglH.
Organizational Affiliation:
Institute of Molecular Biology and Biophysics, Eidgenössische Technische Hochschule (ETH), CH-8093, Zürich, Switzerland.