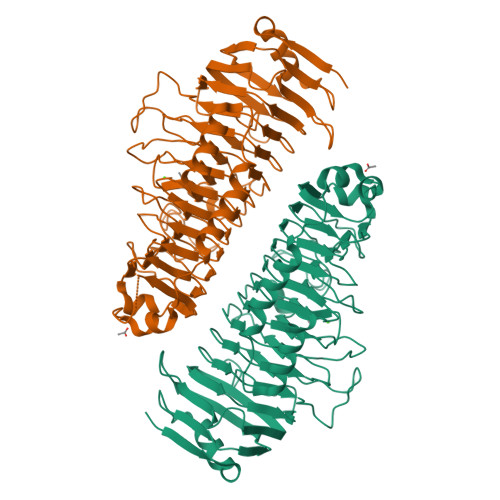
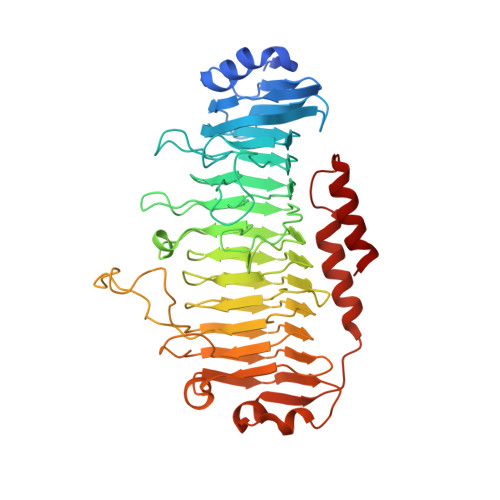
Structural and functional aspects of mannuronic acid-specific PL6 alginate lyase from the human gut microbeBacteroides cellulosilyticus.
Stender, E.G.P., Dybdahl Andersen, C., Fredslund, F., Holck, J., Solberg, A., Teze, D., Peters, G.H.J., Christensen, B.E., Aachmann, F.L., Welner, D.H., Svensson, B.(2019) J Biological Chem 294: 17915-17930
- PubMed: 31530640
- DOI: https://doi.org/10.1074/jbc.RA119.010206
- Primary Citation of Related Structures:
6QPS - PubMed Abstract:
Alginate is a linear polysaccharide from brown algae consisting of 1,4-linked β-d-mannuronic acid (M) and α-l-guluronic acid (G) arranged in M, G, and mixed MG blocks. Alginate was assumed to be indigestible in humans, but bacteria isolated from fecal samples can utilize alginate. Moreover, genomes of some human gut microbiome-associated bacteria encode putative alginate-degrading enzymes. Here, we genome-mined a polysaccharide lyase family 6 alginate lyase from the gut bacterium Bacteroides cellulosilyticus ( Bcel PL6). The structure of recombinant Bcel PL6 was solved by X-ray crystallography to 1.3 Å resolution, revealing a single-domain, monomeric parallel β-helix containing a 10-step asparagine ladder characteristic of alginate-converting parallel β-helix enzymes. Substitutions of the conserved catalytic site residues Lys-249, Arg-270, and His-271 resulted in activity loss. However, imidazole restored the activity of Bcel PL6-H271N to 2.5% that of the native enzyme. Molecular docking oriented tetra-mannuronic acid for syn attack correlated with M specificity. Using biochemical analyses, we found that Bcel PL6 initially releases unsaturated oligosaccharides of a degree of polymerization of 2-7 from alginate and polyM, which were further degraded to di- and trisaccharides. Unlike other PL6 members, Bcel PL6 had low activity on polyMG and none on polyG. Surprisingly, polyG increased Bcel PL6 activity on alginate 7-fold. LC-electrospray ionization-MS quantification of products and lack of activity on NaBH 4 -reduced octa-mannuronic acid indicated that Bcel PL6 is an endolyase that further degrades the oligosaccharide products with an intact reducing end. We anticipate that our results advance predictions of the specificity and mode of action of PL6 enzymes.
Organizational Affiliation:
Department of Biotechnology and Biomedicine, Technical University of Denmark, DK-2800 Kgs. Lyngby, Denmark.