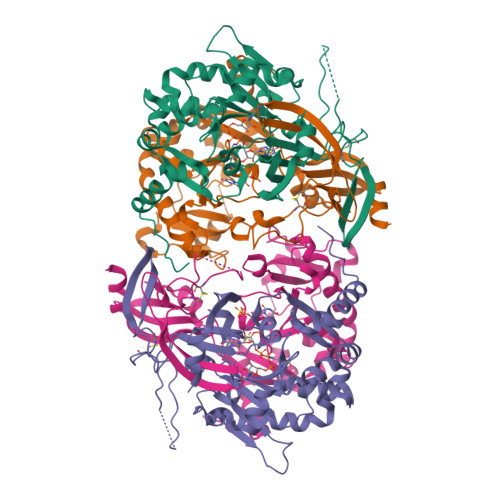
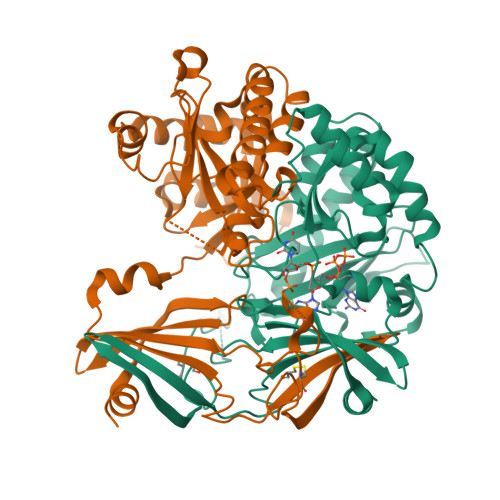
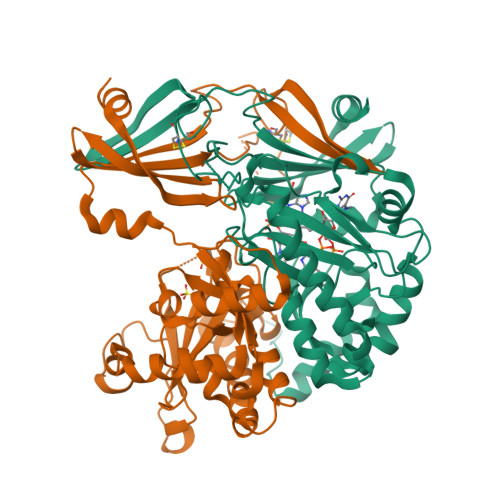
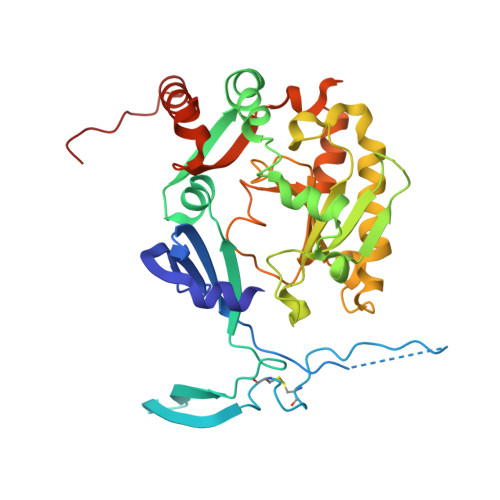
Molecular basis of the selective processing of short mRNA substrates by the DcpS mRNA decapping enzyme.
Fuchs, A.L., Wurm, J.P., Neu, A., Sprangers, R.(2020) Proc Natl Acad Sci U S A 117: 19237-19244
- PubMed: 32723815
- DOI: https://doi.org/10.1073/pnas.2009362117
- Primary Citation of Related Structures:
6GBS, 6TRQ - PubMed Abstract:
The 5' messenger RNA (mRNA) cap structure enhances translation and protects the transcript against exonucleolytic degradation. During mRNA turnover, this cap is removed from the mRNA. This decapping step is catalyzed by the Scavenger Decapping Enzyme (DcpS), in case the mRNA has been exonucleolyticly shortened from the 3' end by the exosome complex. Here, we show that DcpS only processes mRNA fragments that are shorter than three nucleotides in length. Based on a combination of methyl transverse relaxation optimized (TROSY) NMR spectroscopy and X-ray crystallography, we established that the DcpS substrate length-sensing mechanism is based on steric clashes between the enzyme and the third nucleotide of a capped mRNA. For longer mRNA substrates, these clashes prevent conformational changes in DcpS that are required for the formation of a catalytically competent active site. Point mutations that enlarge the space for the third nucleotide in the mRNA body enhance the activity of DcpS on longer mRNA species. We find that this mechanism to ensure that the enzyme is not active on translating long mRNAs is conserved from yeast to humans. Finally, we show that the products that the exosome releases after 3' to 5' degradation of the mRNA body are indeed short enough to be decapped by DcpS. Our data thus directly confirms the notion that mRNA products of the exosome are direct substrates for DcpS. In summary, we demonstrate a direct relationship between conformational changes and enzyme activity that is exploited to achieve substrate selectivity.
Organizational Affiliation:
Department of Biophysics I, Regensburg Center for Biochemistry, University of Regensburg, 93053 Regensburg, Germany.