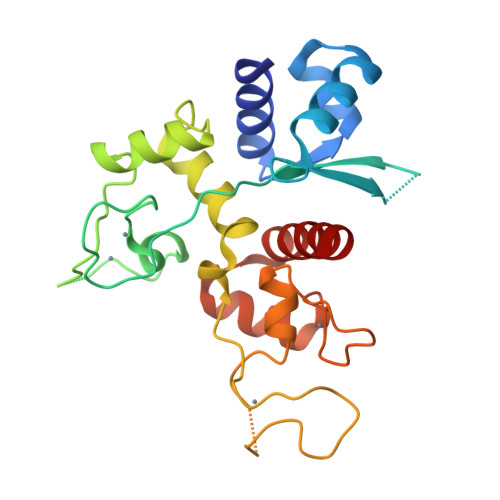
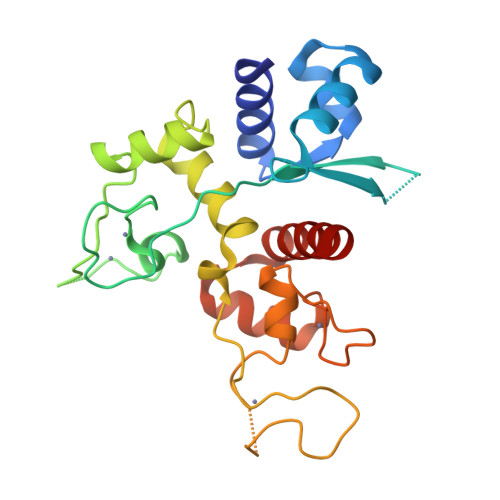
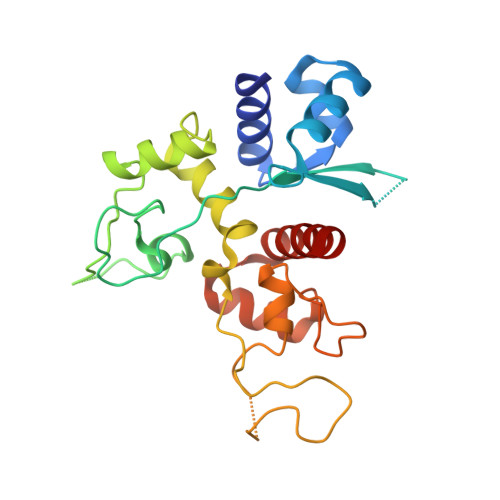
Structure of the zinc-finger antiviral protein in complex with RNA reveals a mechanism for selective targeting of CG-rich viral sequences.
Meagher, J.L., Takata, M., Goncalves-Carneiro, D., Keane, S.C., Rebendenne, A., Ong, H., Orr, V.K., MacDonald, M.R., Stuckey, J.A., Bieniasz, P.D., Smith, J.L.(2019) Proc Natl Acad Sci U S A 116: 24303-24309
- PubMed: 31719195
- DOI: https://doi.org/10.1073/pnas.1913232116
- Primary Citation of Related Structures:
6UEI, 6UEJ - PubMed Abstract:
Infection of animal cells by numerous viruses is detected and countered by a variety of means, including recognition of nonself nucleic acids. The zinc finger antiviral protein (ZAP) depletes cytoplasmic RNA that is recognized as foreign in mammalian cells by virtue of its elevated CG dinucleotide content compared with endogenous mRNAs. Here, we determined a crystal structure of a protein-RNA complex containing the N-terminal, 4-zinc finger human (h) ZAP RNA-binding domain (RBD) and a CG dinucleotide-containing RNA target. The structure reveals in molecular detail how hZAP is able to bind selectively to CG-rich RNA. Specifically, the 4 zinc fingers create a basic patch on the hZAP RBD surface. The highly basic second zinc finger contains a pocket that selectively accommodates CG dinucleotide bases. Structure guided mutagenesis, cross-linking immunoprecipitation sequencing assays, and RNA affinity assays show that the structurally defined CG-binding pocket is not required for RNA binding per se in human cells. However, the pocket is a crucial determinant of high-affinity, specific binding to CG dinucleotide-containing RNA. Moreover, variations in RNA-binding specificity among a panel of CG-binding pocket mutants quantitatively predict their selective antiviral activity against a CG-enriched HIV-1 strain. Overall, the hZAP RBD RNA structure provides an atomic-level explanation for how ZAP selectively targets foreign, CG-rich RNA.
Organizational Affiliation:
Life Sciences Institute, University of Michigan, Ann Arbor, MI 48109.