Structure-Based Design of Potent and Orally Active Isoindolinone Inhibitors of MDM2-p53 Protein-Protein Interaction.
Chessari, G., Hardcastle, I.R., Ahn, J.S., Anil, B., Anscombe, E., Bawn, R.H., Bevan, L.D., Blackburn, T.J., Buck, I., Cano, C., Carbain, B., Castro, J., Cons, B., Cully, S.J., Endicott, J.A., Fazal, L., Golding, B.T., Griffin, R.J., Haggerty, K., Harnor, S.J., Hearn, K., Hobson, S., Holvey, R.S., Howard, S., Jennings, C.E., Johnson, C.N., Lunec, J., Miller, D.C., Newell, D.R., Noble, M.E.M., Reeks, J., Revill, C.H., Riedinger, C., St Denis, J.D., Tamanini, E., Thomas, H., Thompson, N.T., Vinkovic, M., Wedge, S.R., Williams, P.A., Wilsher, N.E., Zhang, B., Zhao, Y.(2021) J Med Chem 64: 4071-4088
- PubMed: 33761253
- DOI: https://doi.org/10.1021/acs.jmedchem.0c02188
- Primary Citation of Related Structures:
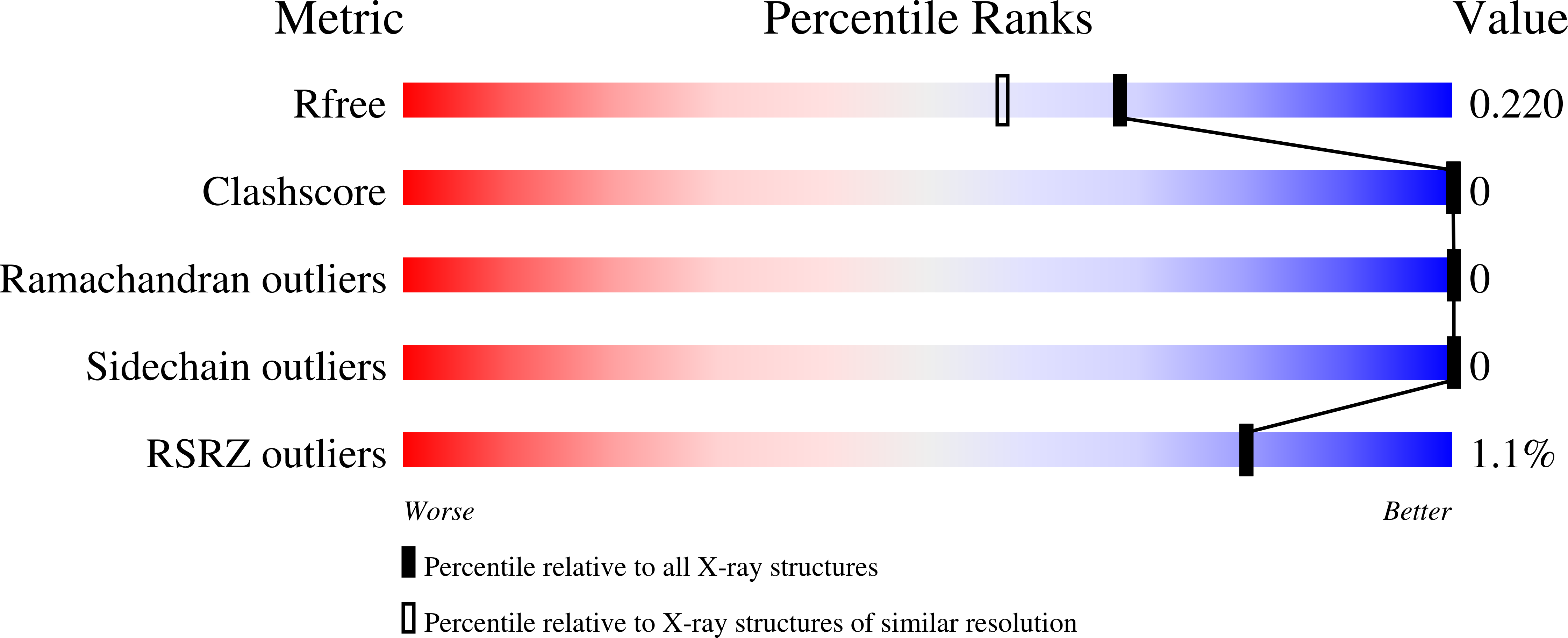
7BIR, 7BIT, 7BIV, 7BJ0, 7BJ6, 7BMG - PubMed Abstract:
Inhibition of murine double minute 2 (MDM2)-p53 protein-protein interaction with small molecules has been shown to reactivate p53 and inhibit tumor growth. Here, we describe rational, structure-guided, design of novel isoindolinone-based MDM2 inhibitors. MDM2 X-ray crystallography, quantum mechanics ligand-based design, and metabolite identification all contributed toward the discovery of potent in vitro and in vivo inhibitors of the MDM2-p53 interaction with representative compounds inducing cytostasis in an SJSA-1 osteosarcoma xenograft model following once-daily oral administration.
Organizational Affiliation:
Astex Pharmaceuticals, 436 Cambridge Science Park, Milton Road, Cambridge CB4 0QA, U.K.