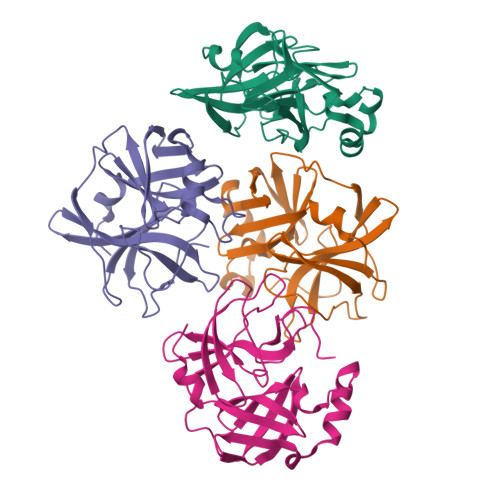
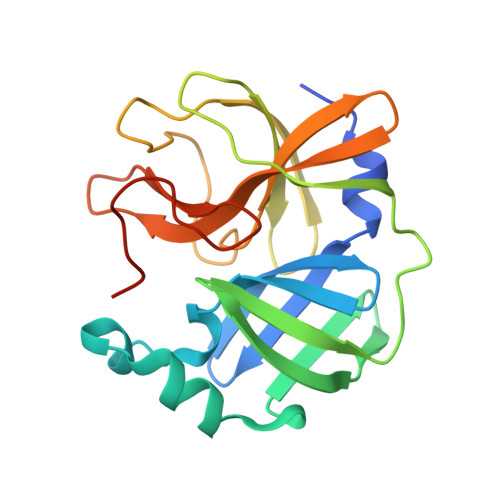
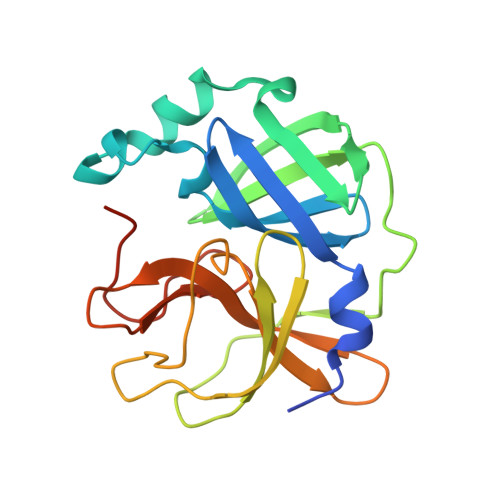
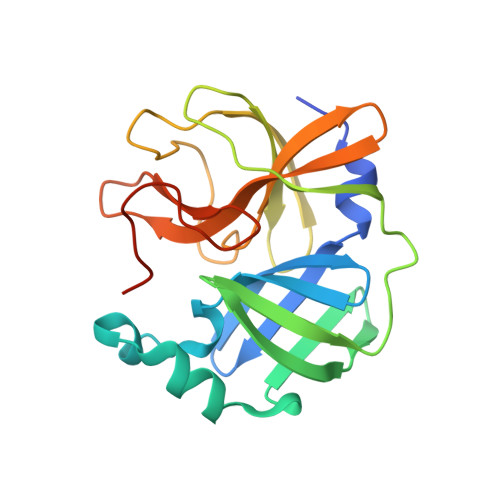
Dimerization Tendency of 3CLpros of Human Coronaviruses Based on the X-ray Crystal Structure of the Catalytic Domain of SARS-CoV-2 3CLpro.
Jo, S., Kim, H.Y., Shin, D.H., Kim, M.S.(2022) Int J Mol Sci 23
- PubMed: 35563658
- DOI: https://doi.org/10.3390/ijms23095268
- Primary Citation of Related Structures:
7WHC - PubMed Abstract:
3CLpro of SARS-CoV-2 is a promising target for developing anti-COVID19 agents. In order to evaluate the catalytic activity of 3CLpros according to the presence or absence of the dimerization domain, two forms had been purified and tested. Enzyme kinetic studies with a FRET method revealed that the catalytic domain alone presents enzymatic activity, despite it being approximately 8.6 times less than that in the full domain. The catalytic domain was crystallized and its X-ray crystal structure has been determined to 2.3 Å resolution. There are four protomers in the asymmetric unit. Intriguingly, they were packed as a dimer though the dimerization domain was absent. The RMSD of superimposed two catalytic domains was 0.190 for 182 Cα atoms. A part of the long hinge loop (LH-loop) from Gln189 to Asp197 was not built in the model due to its flexibility. The crystal structure indicates that the decreased proteolytic activity of the catalytic domain was due to the incomplete construction of the substrate binding part built by the LH-loop. A structural survey with other 3CLpros showed that SARS-CoV families do not have interactions between DM-loop due to the conformational difference at the last turn of helix α7 compared with others. Therefore, we can conclude that the monomeric form contains nascent enzyme activity and that its efficiency increases by dimerization. This new insight may contribute to understanding the behavior of SARS-CoV-2 3CLpro and thus be useful in developing anti-COVID-19 agents.
Organizational Affiliation:
College of Pharmacy and Graduates School of Pharmaceutical Sciences, Ewha Womans University, Seoul 03760, Korea.