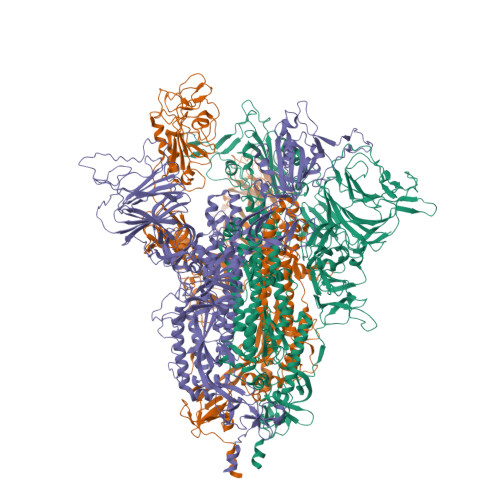
An infectivity-enhancing site on the SARS-CoV-2 spike protein targeted by antibodies.
Liu, Y., Soh, W.T., Kishikawa, J.I., Hirose, M., Nakayama, E.E., Li, S., Sasai, M., Suzuki, T., Tada, A., Arakawa, A., Matsuoka, S., Akamatsu, K., Matsuda, M., Ono, C., Torii, S., Kishida, K., Jin, H., Nakai, W., Arase, N., Nakagawa, A., Matsumoto, M., Nakazaki, Y., Shindo, Y., Kohyama, M., Tomii, K., Ohmura, K., Ohshima, S., Okamoto, T., Yamamoto, M., Nakagami, H., Matsuura, Y., Nakagawa, A., Kato, T., Okada, M., Standley, D.M., Shioda, T., Arase, H.(2021) Cell 184: 3452-3466.e18
- PubMed: 34139176
- DOI: https://doi.org/10.1016/j.cell.2021.05.032
- Primary Citation of Related Structures:
7DZW, 7DZX, 7DZY - PubMed Abstract:
Antibodies against the receptor-binding domain (RBD) of the SARS-CoV-2 spike protein prevent SARS-CoV-2 infection. However, the effects of antibodies against other spike protein domains are largely unknown. Here, we screened a series of anti-spike monoclonal antibodies from coronavirus disease 2019 (COVID-19) patients and found that some of antibodies against the N-terminal domain (NTD) induced the open conformation of RBD and thus enhanced the binding capacity of the spike protein to ACE2 and infectivity of SARS-CoV-2. Mutational analysis revealed that all of the infectivity-enhancing antibodies recognized a specific site on the NTD. Structural analysis demonstrated that all infectivity-enhancing antibodies bound to NTD in a similar manner. The antibodies against this infectivity-enhancing site were detected at high levels in severe patients. Moreover, we identified antibodies against the infectivity-enhancing site in uninfected donors, albeit at a lower frequency. These findings demonstrate that not only neutralizing antibodies but also enhancing antibodies are produced during SARS-CoV-2 infection.
Organizational Affiliation:
Department of Immunochemistry, Research Institute for Microbial Diseases, Osaka University, Osaka 565-0871, Japan; Laboratory of Immunochemistry, World Premier International Immunology Frontier Research Centre, Osaka University, Osaka 565-0871, Japan.