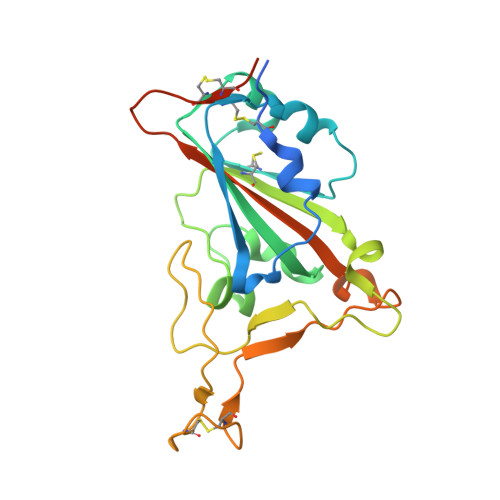
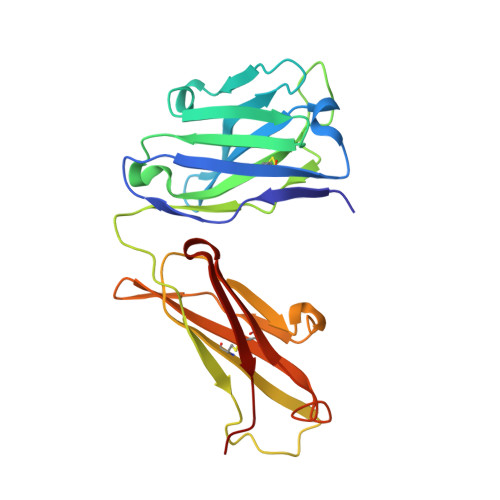
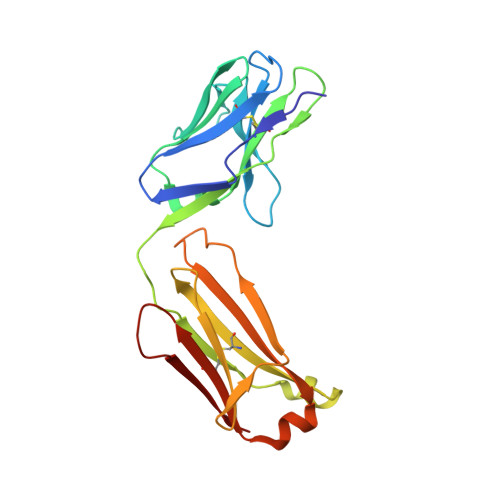
Cross-neutralizing antibodies bind a SARS-CoV-2 cryptic site and resist circulating variants.
Li, T., Xue, W., Zheng, Q., Song, S., Yang, C., Xiong, H., Zhang, S., Hong, M., Zhang, Y., Yu, H., Zhang, Y., Sun, H., Huang, Y., Deng, T., Chi, X., Li, J., Wang, S., Zhou, L., Chen, T., Wang, Y., Cheng, T., Zhang, T., Yuan, Q., Zhao, Q., Zhang, J., McLellan, J.S., Zhou, Z.H., Zhang, Z., Li, S., Gu, Y., Xia, N.(2021) Nat Commun 12: 5652-5652
- PubMed: 34580306
- DOI: https://doi.org/10.1038/s41467-021-25997-3
- Primary Citation of Related Structures:
7EAN - PubMed Abstract:
The emergence of numerous variants of SARS-CoV-2, the causative agent of COVID-19, has presented new challenges to the global efforts to control the COVID-19 pandemic. Here, we obtain two cross-neutralizing antibodies (7D6 and 6D6) that target Sarbecoviruses' receptor-binding domain (RBD) with sub-picomolar affinities and potently neutralize authentic SARS-CoV-2. Crystal structures show that both antibodies bind a cryptic site different from that recognized by existing antibodies and highly conserved across Sarbecovirus isolates. Binding of these two antibodies to the RBD clashes with the adjacent N-terminal domain and disrupts the viral spike. Both antibodies confer good resistance to mutations in the currently circulating SARS-CoV-2 variants. Thus, our results have direct relevance to public health as options for passive antibody therapeutics and even active prophylactics. They can also inform the design of pan-sarbecovirus vaccines.
Organizational Affiliation:
State Key Laboratory of Molecular Vaccinology and Molecular Diagnostics, School of Life Sciences, School of Public Health, Xiamen University, 361102, Xiamen, Fujian, China.