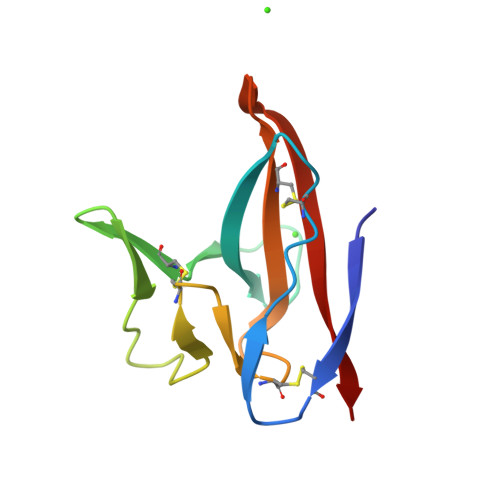
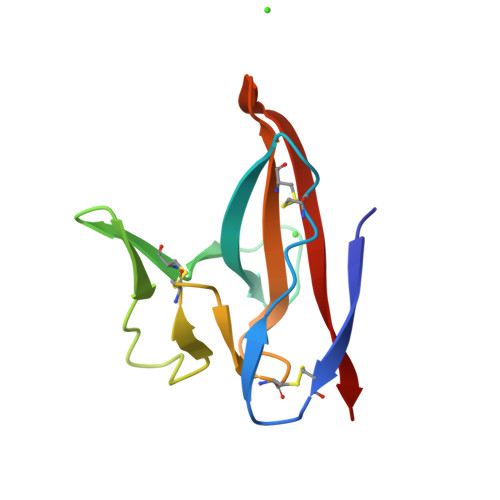
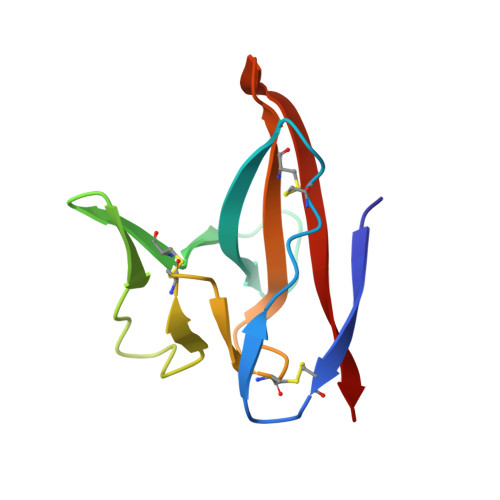
Crystal Structures of Bat and Human Coronavirus ORF8 Protein Ig-Like Domain Provide Insights Into the Diversity of Immune Responses.
Chen, X., Zhou, Z., Huang, C., Zhou, Z., Kang, S., Huang, Z., Jiang, G., Hong, Z., Chen, Q., Yang, M., He, S., Liu, S., Chen, J., Li, K., Li, X., Liao, J., Chen, J., Chen, S.(2021) Front Immunol 12: 807134-807134
- PubMed: 34975921
- DOI: https://doi.org/10.3389/fimmu.2021.807134
- Primary Citation of Related Structures:
7F5F, 7F8L - PubMed Abstract:
ORF8 is a viral immunoglobulin-like (Ig-like) domain protein encoded by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) RNA genome. It tends to evolve rapidly and interfere with immune responses. However, the structural characteristics of various coronavirus ORF8 proteins and their subsequent effects on biological functions remain unclear. Herein, we determined the crystal structures of SARS-CoV-2 ORF8 (S84) (one of the epidemic isoforms) and the bat coronavirus RaTG13 ORF8 variant at 1.62 Å and 1.76 Å resolution, respectively. Comparison of these ORF8 proteins demonstrates that the 62-77 residues in Ig-like domain of coronavirus ORF8 adopt different conformations. Combined with mutagenesis assays, the residue Cys20 of ORF8 is responsible for forming the covalent disulfide-linked dimer in crystal packing and in vitro biochemical conditions. Furthermore, immune cell-binding assays indicate that various ORF8 (SARS-CoV-2 ORF8 (L84), ORF8 (S84), and RaTG13 ORF8) proteins have different interaction capabilities with human CD14 + monocytes in human peripheral blood. These results provide new insights into the specific characteristics of various coronavirus ORF8 and suggest that ORF8 variants may influence disease-related immune responses.
Organizational Affiliation:
Molecular Imaging Center, Guangdong Provincial Key Laboratory of Biomedical Imaging, The Fifth Affiliated Hospital, Sun Yat-sen University, Zhuhai, China.