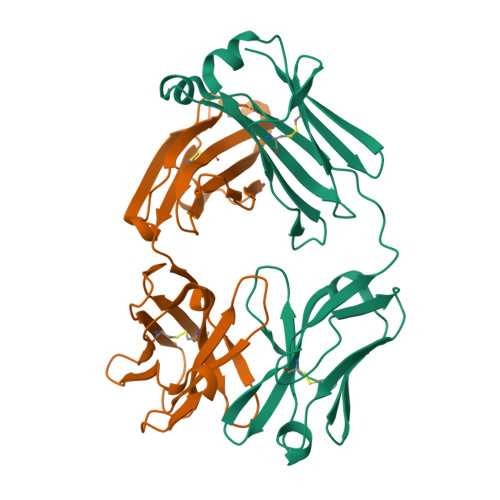
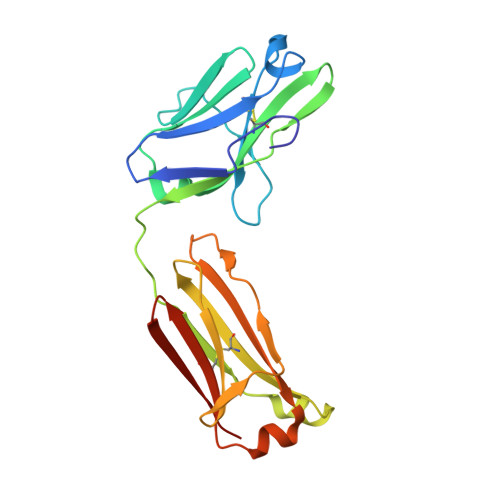
Crystal structure of human immunoglobulin fragment Fab New refined at 2.0 A resolution.
Saul, F.A., Poljak, R.J.(1992) Proteins 14: 363-371
- PubMed: 1438175
- DOI: https://doi.org/10.1002/prot.340140305
- Primary Citation of Related Structures:
7FAB - PubMed Abstract:
The three-dimensional structure of the human immunoglobulin fragment Fab New (IgG1, lambda) has been refined to a crystallographic R-factor of 16.9% to 2 A resolution. Rms deviations of the final model from ideal geometry are 0.014 A for bond distances and 3.03 degrees for bond angles. Refinement was based on a new X-ray data set including 28,301 reflections with F > 2.5 sigma(F) from 6.0 to 2.0 A resolution. The starting model for the refinement procedure reported here is from the Brookhaven Protein Data Bank entry 3FAB (rev. 1981). Differences between the initial and final models include modified polypeptide-chain folding in the third complementarity-determining region (CDR3) and the third framework region (FR3) of VH and in some exposed loops of CL and CH1. Amino acid sequence changes were determined at a number of positions by inspection of difference electron density maps. The incorporation of amino acid sequence changes results in an improved VH framework model for the "humanization" of monoclonal antibodies.
Organizational Affiliation:
Département d'Immunologie, Institut Pasteur, Paris, France.