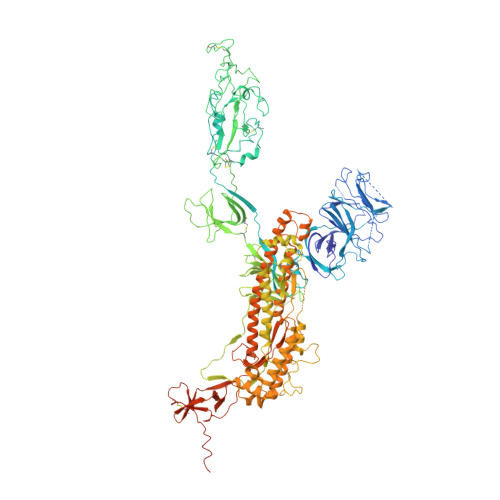
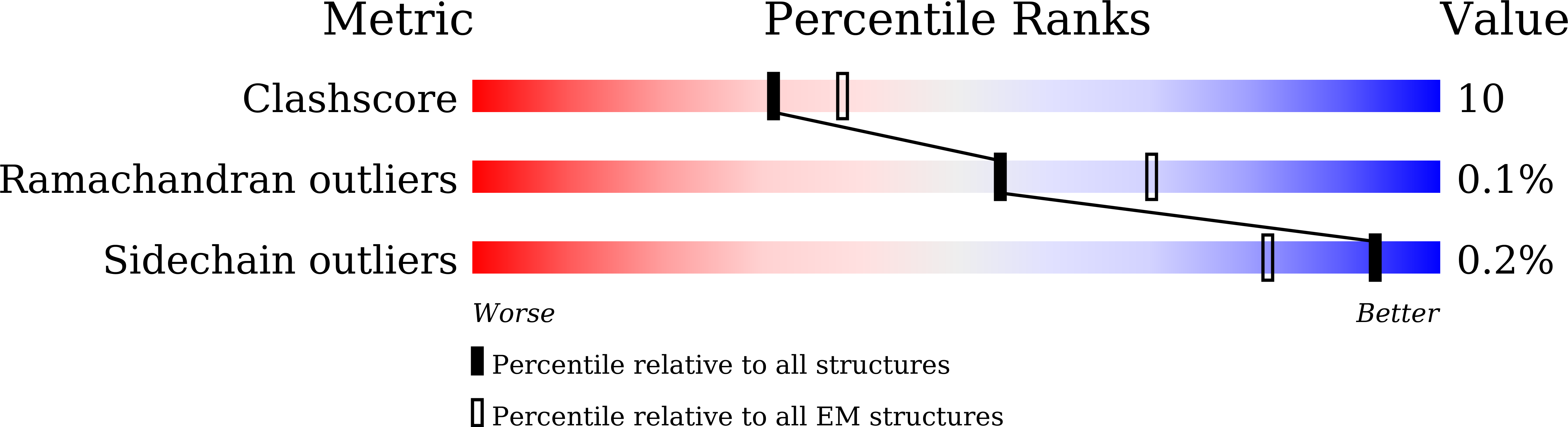Structure-based evidence for the enhanced transmissibility of the dominant SARS-CoV-2 B.1.1.7 variant (Alpha).
Xia, S., Wen, Z., Wang, L., Lan, Q., Jiao, F., Tai, L., Wang, Q., Sun, F., Jiang, S., Lu, L., Zhu, Y.(2021) Cell Discov 7: 109-109
- PubMed: 34750362
- DOI: https://doi.org/10.1038/s41421-021-00349-z
- Primary Citation of Related Structures:
7FEM, 7FET
Organizational Affiliation:
Key Laboratory of Medical Molecular Virology (MOE/NHC/CAMS), School of Basic Medical Sciences and Biosafety Level 3 Laboratory, Shanghai Institute of Infectious Disease and Biosecurity, Fudan University, Shanghai, China.