Discovery of Cyclic Peptide Ligands to the SARS-CoV-2 Spike Protein Using mRNA Display.
Norman, A., Franck, C., Christie, M., Hawkins, P.M.E., Patel, K., Ashhurst, A.S., Aggarwal, A., Low, J.K.K., Siddiquee, R., Ashley, C.L., Steain, M., Triccas, J.A., Turville, S., Mackay, J.P., Passioura, T., Payne, R.J.(2021) ACS Cent Sci 7: 1001-1008
- PubMed: 34230894
- DOI: https://doi.org/10.1021/acscentsci.0c01708
- Primary Citation of Related Structures:
7L4Z - PubMed Abstract:
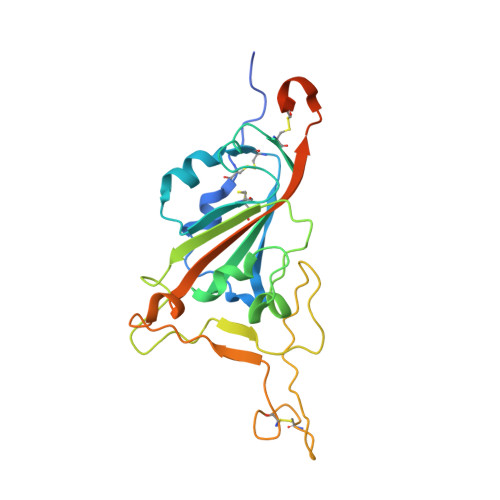
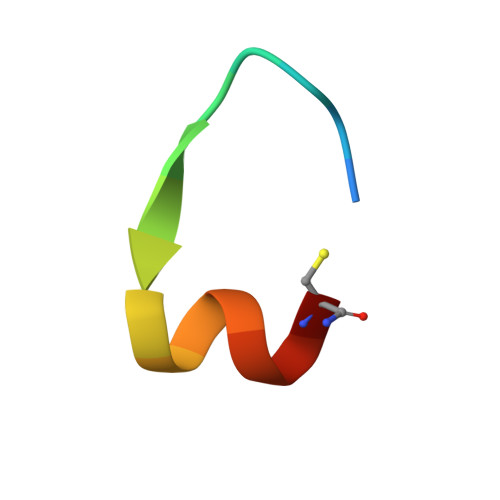
The COVID-19 pandemic, caused by SARS-CoV-2, has led to substantial morbidity, mortality, and disruption globally. Cellular entry of SARS-CoV-2 is mediated by the viral spike protein, and affinity ligands to this surface protein have the potential for applications as antivirals and diagnostic reagents. Here, we describe the affinity selection of cyclic peptide ligands to the SARS-CoV-2 spike protein receptor binding domain (RBD) from three distinct libraries (in excess of a trillion molecules each) by mRNA display. We identified six high affinity molecules with dissociation constants ( K D ) in the nanomolar range (15-550 nM) to the RBD. The highest affinity ligand could be used as an affinity reagent to detect the spike protein in solution by ELISA, and the cocrystal structure of this molecule bound to the RBD demonstrated that it binds to a cryptic binding site, displacing a β-strand near the C-terminus. Our findings provide key mechanistic insight into the binding of peptide ligands to the SARS-CoV-2 spike RBD, and the ligands discovered in this work may find future use as reagents for diagnostic applications.
Organizational Affiliation:
School of Chemistry, The University of Sydney, Sydney, New South Wales 2006, Australia.