B cell genomics behind cross-neutralization of SARS-CoV-2 variants and SARS-CoV.
Scheid, J.F., Barnes, C.O., Eraslan, B., Hudak, A., Keeffe, J.R., Cosimi, L.A., Brown, E.M., Muecksch, F., Weisblum, Y., Zhang, S., Delorey, T., Woolley, A.E., Ghantous, F., Park, S.M., Phillips, D., Tusi, B., Huey-Tubman, K.E., Cohen, A.A., Gnanapragasam, P.N.P., Rzasa, K., Hatziioanno, T., Durney, M.A., Gu, X., Tada, T., Landau, N.R., West Jr., A.P., Rozenblatt-Rosen, O., Seaman, M.S., Baden, L.R., Graham, D.B., Deguine, J., Bieniasz, P.D., Regev, A., Hung, D., Bjorkman, P.J., Xavier, R.J.(2021) Cell 184: 3205
- PubMed: 34015271
- DOI: https://doi.org/10.1016/j.cell.2021.04.032
- Primary Citation of Related Structures:
7M6D, 7M6E, 7M6F, 7M6G, 7M6H, 7M6I - PubMed Abstract:
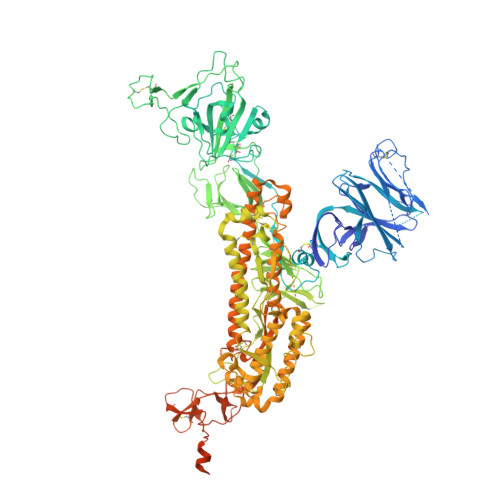
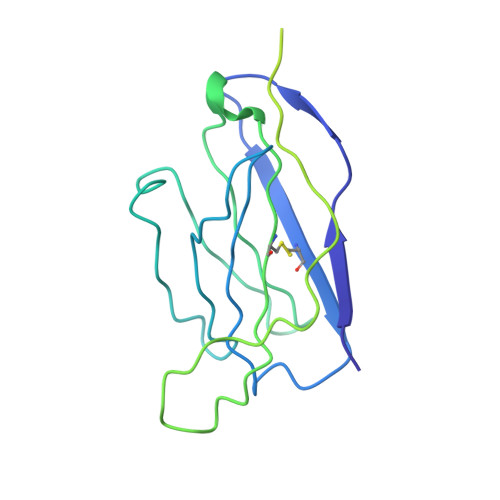
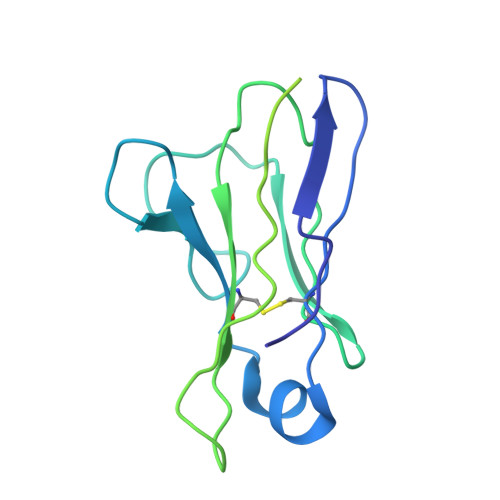
Monoclonal antibodies (mAbs) are a focus in vaccine and therapeutic design to counteract severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and its variants. Here, we combined B cell sorting with single-cell VDJ and RNA sequencing (RNA-seq) and mAb structures to characterize B cell responses against SARS-CoV-2. We show that the SARS-CoV-2-specific B cell repertoire consists of transcriptionally distinct B cell populations with cells producing potently neutralizing antibodies (nAbs) localized in two clusters that resemble memory and activated B cells. Cryo-electron microscopy structures of selected nAbs from these two clusters complexed with SARS-CoV-2 spike trimers show recognition of various receptor-binding domain (RBD) epitopes. One of these mAbs, BG10-19, locks the spike trimer in a closed conformation to potently neutralize SARS-CoV-2, the recently arising mutants B.1.1.7 and B.1.351, and SARS-CoV and cross-reacts with heterologous RBDs. Together, our results characterize transcriptional differences among SARS-CoV-2-specific B cells and uncover cross-neutralizing Ab targets that will inform immunogen and therapeutic design against coronaviruses.
Organizational Affiliation:
Broad Institute of the Massachusetts Institute of Technology and Harvard University, Cambridge, MA 02142, USA; Division of Gastroenterology, Massachusetts General Hospital and Harvard Medical School, Boston, MA 02114, USA; Center for Computational and Integrative Biology, Massachusetts General Hospital and Harvard Medical School, Boston, MA 02114, USA.